 |
 |
| Korean J Intern Med > Volume 35(3); 2020 > Article |
|
Abstract
Background/Aims
Seasonal variation is an environmental factor proposed to affect the incidence of venous thromboembolism (VTE). However, VTE seasonal variation is not well studied in Asian populations, which have different genetic determinants of VTE compared to Westerners. The present study aimed at investigating seasonal variation of VTE occurrence and the effect of various demographic factors (i.e., age, sex, and co-morbidities) on variation.
Methods
VTE seasonal variation was evaluated in 59,626 index cases (from January 2009 to December 2013) in the Korean Health Insurance Review and Assessment Service database. We quantified and compared VTE occurrence across four seasons, and additionally assessed monthly through a chronobiological analysis.
Results
VTE incidence varied both seasonally and monthly, with new cases peaking in the winter (January and February) and the lowest incidence in the summer (August and September). After adjusting for sex, age, type of VTE, and combined cancer diagnosis, winter remained a significant independent factor driving VTE incidence. Additionally, seasonal variation was prominent in patients aged 60 years or older and in patients with pulmonary embolism, but not so prominent in patients of aged less than 60 years and patients with deep vein thrombosis.
Venous thromboembolism (VTE) occurs when a blood clot breaks loose and travels freely in the blood. Deep vein thrombosis (DVT) is a blood clot in a deep vein, most often in the lower extremities, which may break off and travel to the lungs, causing a pulmonary embolism (PE). PEs can cause acute cardiopulmonary collapse and death [1], and, when not fatal, may be associated with chronic complications such as post-thrombotic syndrome and chronic thromboembolic pulmonary hypertension [2]. The social and economic costs associated with VTE are significant [3] and lead to decreased quality of life for patients and caretakers. Therefore, it is critical to identify factors contributing to increased VTE incidence to improve monitoring of those at risk and to enable proper and timely prevention and treatment.
Several VTE risk factors have been identified and include old age, inherited thrombophilia, surgery, and cancer and other comorbidities; additionally, VTE is more common in women than in men [4]. Because many of these factors are unchangeable and unavoidable, identifying and leveraging environmental factors contributing to VTE likely represents a better approach for VTE prediction and prevention. Seasonal variation is a proposed environmental factor associated with VTE incidence, with several studies reporting a seasonal or monthly variation in the incidence of VTE [5-16]. However, some studies failed to detect an association between season and VTE [17] or reported only a small association [18]. These results may be due to differences in the definition of seasonal or monthly variation across studies; however, differences in annual climate patterns and geography as well as ethnicity (and therefore, genetics) may play a role. For example, the genetic factors associated with VTE in Asian populations are fundamentally different from those in Western populations [19,20].
Whether seasonal variation affects VTE in Asian populations remains unclear. A previous retrospective study of 1,495 Korean VTE patients indeed reported an increased risk of VTE in winter [21]; however, the number of patients evaluated was relatively small, and over 90% of patients were inpatient cases diagnosed in tertiary teaching hospitals [21]. We therefore conducted a nationwide epidemiologic study to evaluate the extent of seasonal and monthly variation in VTE incidence among the Korean population from January 2009 to December 2013. We also evaluated the impact of age, sex, cancer, and thromboembolism type on seasonal and monthly VTE variation to fully elucidate the factors affecting VTE seasonal variation.
With the exception of about 3% of the population, Republic of Korea nationals that reside in the Republic of Korea are subject to a single public health care insurer, the National Health Insurance Service (NHIS; the other 3% are covered by the National Medical Aid program). In 2013, the number of peopled registered by the NHIS totaled 49,989,620, including 645,828 foreign residents and Koreans travelling abroad that were also covered by the NHIS. Health care providers, whether hospitals or outpatient clinics, whether public or private institutions, submit claims data to the Health Insurance Review and Assessment Service (HIRA), a government-owned organization established for building a proper review an d quality assessment system for NHIS claims. Claims data submitted to HIRA include patients’ age, sex, diagnosis codes, and prescribed medications, procedures or surgeries. Because claims data from individuals covered by the National Medical Aid program (1,458,871 in 2013) are also reviewed by HIRA, the HIRA database is a powerful resource that enables essentially nationwide epidemiologic studies [22].
Ethical review of the current study and informed consent from study subjects were exempted from the Institutional Review Board of Seoul National University Bundang Hospital because this study used public information and did not collect or record personally identifiable data (IRB exemption number: X-1608/360-901). The use of HIRA database is regulated by the Rules for Data Exploration and Utilization of the HIRA, and our study was reviewed and approved by the HIRA data access committee (Big Data Division, Healthcare Data Convergence Department, HIRA, Wonju, Korea). All data were anonymized prior to delivery to remove personally identifying information.
For this study, we defined VTE as PE and/or DVT in a lower extremity. Patients with DVT in upper extremities or splanchnic veins were excluded. If a patient had both DVT and PE, the patient was categorized as a PE patient. Only primary VTE events, diagnosed between January 2009 and December 2013, were included (recurring VTE episodes were excluded) in the current study. To ensure accurate identification of treated VTE cases, we required both a diagnostic code for VTE and a medication code for anticoagulants in the HIRA database record. Accepted medication codes were codes for unfractionated heparin, low-molecular weight heparin, or direct oral anticoagulants. Medication codes for warfarin was not included for the initial identification of VTE patients but was subsequently monitored in the follow-up of treatment of the patients. Detailed methods regarding the selection of patients who were diagnosed as VTE from January 2009 to December 2013 and are summarized in Fig. 1 and explained in our previous study [23].
Diagnosis season was categorized according to the general distribution of four seasons in Korea: March to May, spring; June to August, summer; September to November, autumn; and December to February, winter.
The incidence of VTE in the Korean population from January 2009 to December 2013 was determined by dividing the number of individuals with DVT and/or PE by the total number individuals registered in the HIRA database. We quantified VTE diagnoses for each of the four seasons and cross-compared seasonal incidence using a chi-square test. Because the number of days in a month varies from 28 to 31, we calculated VTE incidence ratios adjusted for the numbers of days. A poisson regression analysis adjusted for age, sex, and co-morbidities confirmed seasonal differences in VTE incidence.
We additionally assessed monthly VTE incidence variation using the R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) package ‘season,’ applying a generalized linear model with month (GLMM) as the categorical variable.
A total of 59,626 index VTE cases occurring between January 2009 and December 2013 were identified and classified into one of four seasons. Overall, the number of VTE diagnoses was significantly higher in winter compared to other seasons (p < 0.001), with the largest difference between winter and summer (10.1% higher) (Table 1). When accounting for demographic factors, seasonal differences in VTE incidence were most prominent in females (p < 0.001), patients age 60 years or greater (p < 0.001), patients without cancer (p < 0.001), and patients with PE (p < 0.001). In contrast, the difference was less prominent in males (p = 0.004) and in patients with cancer (p = 0.002). No significant seasonal differences were observed in patients less than 60 years old (p = 0.647), or in patients with DVT (p = 0.071) (Table 1).
Adjusting for age (greater than 60 years vs. less than 60 years), sex, type of VTE (PE vs. DVT) and cancer diagnosis in a multivariate Poisson regression analysis revealed the winter season as an independent factor associated with significantly higher VTE incidence (Table 2). Among patients with PE, the incidence in winter season was significantly higher than in spring (p < 0.001), summer (p < 0.001), and autumn (p < 0.001). Among patients with DVT, the incidence in winter was significantly higher than that in autumn (p = 0.015) but only marginally higher than that in spring (p = 0.058) and was not significantly higher than that in summer (p = 0.374).
A plot of the generalized linear model with month as the categorical variable revealed monthly variation in addition to seasonal variation (Figs. 2 and 3). VTE incidence was highest in January and February but lowest in August and September, agreeing with our previous observation that VTE incidence is higher in winter. Similar to our seasonal analysis, monthly variation was apparent in PE patients and in patients 60 years old or older but was not observed in patients with DVT or less than 60 years of age (Figs. 4 and 5). Monthly variations were apparent in males and females and in patients with and without cancer, and the variation was more prominent in VTE patients without cancer. Figs. 3 and 5 show that the incidence of VTE is going up with time, as we defined in a recent epidemiologic study [23].
Here, using data available in a nationwide database, we demonstrate seasonal and monthly variation in VTE incidence in the Korean population. We assessed NHIS claims records for over 59,000 VTE patients, making this the largest study of its kind in an Asian population. Our results agree with several other studies [5,6,8,9,11,14] and a meta-analysis [16] in other populations also reporting an association between seasonal climate change and VTE incidence. We specifically observed highest incidence of VTE in the winter months, which may be due to a thrombophilia caused by increased fibrinogen and red blood cell aggregation in the cold environment [24]. Seasonal variation has also been pathophysiologically explained by the circannual rhythm (endogenously generated, year-long biological rhythms), which has been proposed in other systemic vascular disorders [25-28].
The only other study in another population that rivals the size of ours is a United States-based study comprised of 7,682,000 VTE patients diagnosed between 1979 and 1999 [17]. Unlike our study and many others, VTE incidence was not associated with season. These contrasting results were previously partly explained by the usage of different analysis methods across studies [29,30]. Additionally, the United States study is older than most; therefore, although the number of patients was large, they were diagnosed mostly in 1980s and 1990s, which likely does not reflect the current medical environment with improved early screening and diagnostic techniques for VTE. Our study, which is large-scale, better reflects the current medical landscape, and takes place in a geographical region with sharp seasonal climate differences therefore yields more reliable and realistic conclusions regarding seasonal variation of VTE incidence.
In our study, seasonal variation was stronger in patients with PE, with a particularly strong association between PE incidence and the winter season. This result aligns with other studies reporting seasonable variability for other potentially fatal vascular conditions, such as myocardial infarction [25,28], stroke [26], and aortic dissection [27], as well as with several reports in the 1990s describing a higher incidence of fatal PE in winter [5,7,8,13]. Several studies have also reported seasonal variation in the incidence of non-fatal PE [10,12]. Moreover, recently published studies showed that the seasonal variation of PE incidence was affected by age of patients [11,14], in line with our study, and suggest that for younger patients, other factors including inherited thrombophilia or diagnosis of cancer may have a stronger impact on the incidence of PE.
The strong association between season and PE incidence may have several explanatory mechanisms. First, inhalation of cold air produces a considerable increase in blood viscosity [31], increasing the risk for thrombophilia during the cold winter season [32]. Second, pulmonary infections and aggravation of pulmonary diseases such as chronic pulmonary obstructive disease are more common in winter [33]. Inflammation of pulmonary blood vessels caused by these conditions may provoke de novo PE or complicate recovery from DVT-caused pulmonary vascular clots. Third, sepsis also occurs more frequently in winter [34], and considering that the surface area of the pulmonary vasculature is larger than that of the lower extremities, pulmonary inflammation may be more likely to facilitate PE. Finally, during winter season air pollutants are elevated [35], particularly in big cities, and this may be responsible for the seasonal variation of PE, as in other cardiovascular diseases [36]. Future studies considering these factors will be critical to fully characterize the relationship between season and PE incidence and inform clinicians regarding best practices for early PE detection at different times of year.
Unlike PE, reports of seasonal variation in DVT incidence are conflicting. Some early studies reported no seasonal or monthly variation in patients with DVT, but they also did not indicate whether DVT patients had concomitant PE or not, which may skew results [6,37]. An Italian study of 1,164 consecutive cases of DVT reported at a single institution from 1998 to 2002 revealed a peak in DVT incidence in winter, with a lower incidence in summer [9]; however, 793 patients (68.1%) had concomitant PE a variable which was not controlled for. The Italian multicenter MASTER study (January 2002 to November 2004 [32]) did evaluate DVT and PE seasonal variability separately in 2,119 VTE patients (72.7% of patients had DVT alone) reporting seasonal variation in the incidence of both VTE and PE, with peak incidence in winter and lowest incidence in summer [38]. Similar observations were reported using data from the Swedish Hospital Discharge Register from 1987 to 2010, but a family history of VTE was a confounding factor when considering seasonal variation in DVT incidence [14]. In our study, we consider DVT and PE separately, and observed a weak, insignificant association between season and DVT incidence. The association was even weaker in younger patients and in those with cancer, suggesting that seasonal variation is a relatively weak contributor to VTE occurrence compared to inherited thrombophilia or combined malignancy. This observation supports a nationwide Swedish family study in Sweden [14]; however, doctors and patients should not assume that these results indicate that young patients or patients with cancer should dismiss the risk of VTE in winter.
Detection of low-burden, isolated DVT has improved drastically, which may explain the less prominent seasonal variation in patients with DVT alone, and we propose that it can be assumed that earlier stage, minimally or asymptomatic DVT can occur without the influence of seasonal change. For those with earlier staged DVT, which often presents as DVT alone, factors other than seasonal variation seem to be more influential on DVT incidence. Further studies are needed to evaluate these potential explanations for the lack of seasonal variation in DVT incidence.
Our study has several limitations. First, the information in the HIRA database was insufficient to discern between provoked and unprovoked VTE or to sub-divide patients into of VTE into inpatient or outpatient. Because we collected VTE patients with cancer according to having diagnostic code for cancer at the time of diagnosis, we could not clearly discern actual status of cancer for each patient. We could not evaluate provoking conditions such as surgery, bone fracture, or pregnancy. However, such provoking factors themselves are not likely to occur in particular seasons. If DVT is diagnosed first in practice then co-existing PE was confirmed later, physician may not have registered additional diagnostic code for PE. For this reason, there is a risk of overestimation of the incidence of isolated DVT. Despite some of these limitations, our study clearly demonstrated seasonal variation in overall VTE incidence in the Korean population and identified sub-groups for which this association is strongest. This study would be a springboard to further studies designed to inform improved VTE detection, prevention, and patient education in Korea and Asian population.
1. Seasonal variation was a weak but clear and independent contributor to venous thromboembolism (VTE) incidence in Korean population diagnosed VTE from 2009 to 2013.
2. The seasonal variation was more prominent in patients with pulmonary embolism, older age, and without a diagnosis of cancer, compared to those with deep vein thrombosis, younger age, and with a diagnostic code of cancer, respectively.
Acknowledgments
This work was supported by grants from the Korean Society on Thrombosis and Haemostasis (KSTH 2016- 002) and Seoul National University Bundang Hospital (04-2012-003).
Figure 1.
Selection of index venous thromboembolism (VTE) patients from January 2009 to December 2013. DVT, deep vein thrombosis; PE, pulmonary embolism; UFH, unfractionated heparin; LMWH, low molecular weight heparin.
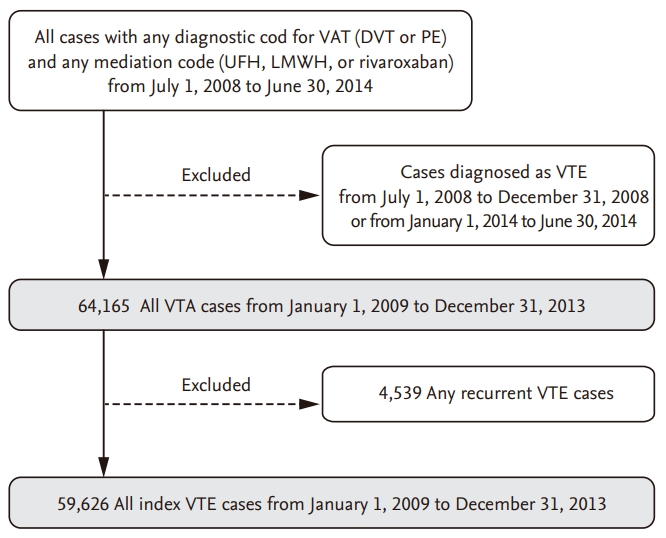
Figure 2.
The relative risks and 95% confidence intervals from Poisson model with month as a categorical variable and January as a reference month.
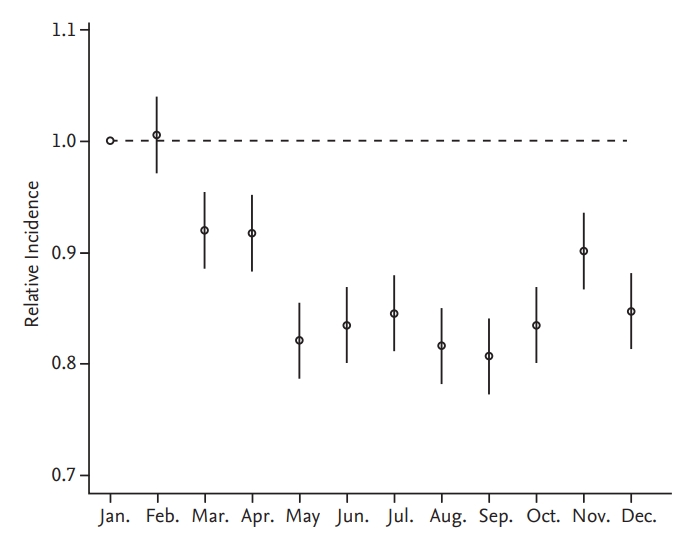
Figure 3.
Trends of the monthly venous thromboembolism (VTE) occurrence from January 2009 to December 2013. The difference in the number of days for each month was statistically corrected.
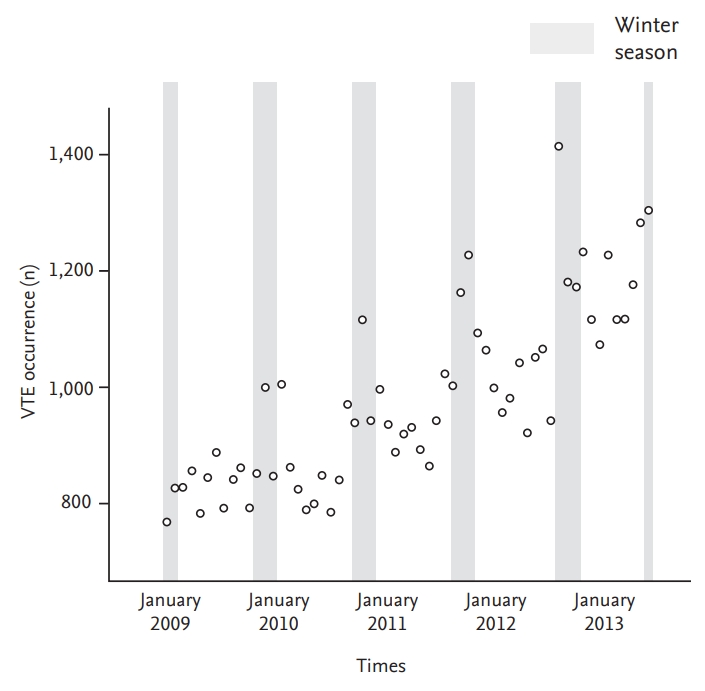
Figure 4.
Plots of the generalized linear model with month as the categorical variable, per subgroup. The incidence in January is the reference (1.0) for the relative incidence of each other month. (A) Age < 60 years, (B) age ≥ 60 years, (C) male, (D) female, (E) cancer, (F) non-cancer, (G) deep vein thrombosis, (H) pulmonary embolism.
Figure 5.
Trends of the monthly venous thromboembolism occurrence from January 2009 to December 2013 in patients with (A) pulmonary embolism, (B) deep vein thromsis, (C) age ≥ 60 years, and (D) age < 60 years. The difference in the number of days for each month was statistically corrected.
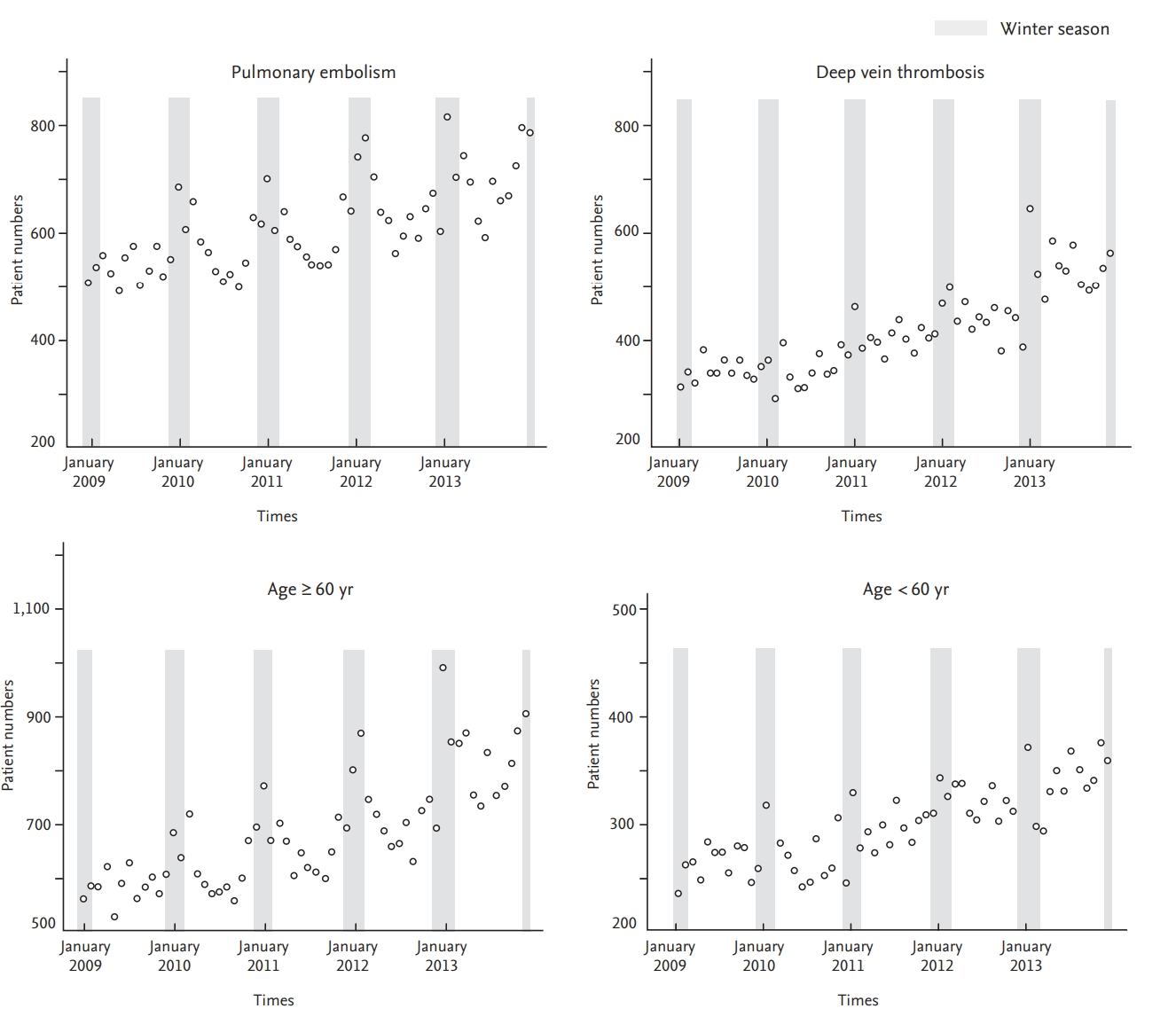
Table 1.
Comparison of VTE incidence across four seasons stratified by sex, age, cancer, and VTE type subgroups
| Parameter | Total | Spring | Summer | Autumn | Winter | Chi-squarea | p value | Ratiob, winter/summer, %a |
|---|---|---|---|---|---|---|---|---|
| Total | 59,626 (23.12)c | 14,975 (5.76)d | 14,412 (5.54)d | 14,709 (5.72)d | 15,530 (6.10)d | 74.242 | < 0.001 | 110.1 |
| Male | 27,303 (21.10)c | 6,934 (5.32)d | 6,657 (5.10)d | 6,790 (5.26)d | 6,922 (5.42)d | 13.125 | 0.004 | 106.2 |
| Female | 32,323 (25.14)c | 8,041 (6.20) d | 7,755 (5.98)d | 7,919 (6.18)d | 8,608 (6.78)d | 74.546 | < 0.001 | 113.4 |
| Age < 60 yr | 17,451 (8.03)c | 4,368 (1.99) d | 4,432 (2.02)d | 4,399 (2.03)d | 4,252 (1.98)d | 1.656 | 0.647 | 97.9 |
| Age ≥ 60 yr | 42,175 (103.67)c | 10,607 (25.86) d | 9,980 (24.32)d | 10,310 (25.41)d | 11,278 (28.08)d | 120.125 | < 0.001 | 115.5 |
| With cancer | 19,725 (1,831.01)c | 4,898 (405.07)d | 4,803 (442.22)d | 4,974 (406.03)d | 5,050 (474.70)d | 14.388 | 0.002 | 107.3 |
| Without cancer | 39,901 (15.54)c | 10,077 (3.89)d | 9,609 (3.71)d | 9,735 (3.80)d | 10,480 (4.13)d | 67.373 | < 0.001 | 111.4 |
| Deep vein thrombosise | 23,800 | 5,929 | 6,046 | 5,808 | 6,017 | 7.04 | 0.071 | 101.6 |
| Pulmonary embolisme | 35,826 | 9,046 | 8,366 | 8,901 | 9,513 | 99.48 | < 0.001 | 116.2 |
Table 2.
Result of multivariate poisson regression analysis of seasonality and VTE, DVT, and PE
| Subgroup |
Venous thromboembolism |
Deep vein thrombosisa |
Pulmonary embolisma |
||||||
|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | p value | RR | 95% CI | p value | RR | 95% CI | p value | |
| Sex | |||||||||
| Male | 1.00 | 1.00 | 1.00 | ||||||
| Female | 1.20 | 1.18–1.22 | < 0.001 | 1.18 | 1.15–1.21 | < 0.001 | 1.22 | 1.20–1.25 | < 0.001 |
| Age, yr | |||||||||
| < 60 | 1.00 | 1.00 | 1.00 | ||||||
| ≥ 60 | 8.42 | 8.26–8.58 | < 0.001 | 6.07 | 5.90–6.26 | < 0.001 | 10.67 | 10.41–10.95 | < 0.001 |
| Association to cancer | |||||||||
| Non-cancer | 1.00 | 1.00 | 1.00 | ||||||
| Cancer | 53.25 | 52.30–54.23 | < 0.001 | 58.20 | 56.53–59.91 | < 0.001 | 50.39 | 49.23–51.57 | |
| Season | |||||||||
| Winter | 1.00 | 1.00 | 1.00 | ||||||
| Spring | 0.94 | 0.92–0.97 | < 0.001 | 0.97 | 0.93–1.00 | 0.058 | 0.93 | 0.90–0.95 | < 0.001 |
| Summer | 0.91 | 0.89–0.93 | < 0.001 | 0.98 | 0.95–1.02 | 0.374 | 0.86 | 0.84–0.89 | < 0.001 |
| Autumn | 0.94 | 0.92–0.96 | < 0.001 | 0.96 | 0.92–0.99 | 0.015 | 0.93 | 0.90–0.96 | < 0.001 |
REFERENCES
2. Winter MP, Schernthaner GH, Lang IM. Chronic complications of venous thromboembolism. J Thromb Haemost 2017;15:1531–1540.


3. Ruppert A, Steinle T, Lees M. Economic burden of venous thromboembolism: a systematic review. J Med Econ 2011;14:65–74.


4. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003;107(23 Suppl 1):I9–I16.


5. Allan TM, Douglas AS. Seasonal variation in deep vein thrombosis. Fatal pulmonary embolism is increased in both autumn and winter. BMJ 1996;312:1227.

6. Bounameaux H, Hicklin L, Desmarais S. Seasonal variation in deep vein thrombosis. BMJ 1996;312:284–285.



7. Chau KY, Yuen ST, Wong MP. Seasonal variation in the necropsy incidence of pulmonary thromboembolism in Hong Kong. J Clin Pathol 1995;48:578–579.



8. Colantonio D, Casale R, Natali G, Pisqualetti P. Seasonal periodicity in fatal pulmonary thromboembolism. Lancet 1990;335:56–57.

9. Gallerani M, Boari B, de Toma D, Salmi R, Manfredini R. Seasonal variation in the occurrence of deep vein thrombosis. Med Sci Monit 2004;10:CR191–CR196.

10. Manfredini R, Gallerani M, Boari B, Salmi R, Mehta RH. Seasonal variation in onset of pulmonary embolism is independent of patients' underlying risk comorbid conditions. Clin Appl Thromb Hemost 2004;10:39–43.


11. Olie V, Bonaldi C. Pulmonary embolism: does the seasonal effect depend on age? A 12-year nationwide analysis of hospitalization and mortality. Thromb Res 2017;150:96–100.


12. Sharma GV, Frisbie JH, Tow DE, Yalla SV, Khuri SF. Circadian and circannual rhythm of nonfatal pulmonary embolism. Am J Cardiol 2001;87:922–924.


13. Silcocks P. Seasonal variation in numbers of necropsies with pulmonary embolism. J Clin Pathol 1994;47:478–479.

14. Zoller B, Li X, Ohlsson H, Sundquist J, Sundquist K. Ageand sex-specific seasonal variation of venous thromboembolism in patients with and without family history: a nationwide family study in Sweden. Thromb Haemost 2013;110:1164–1171.



15. Ferrari E, Baudouy M, Cerboni P, et al. Clinical epidemiology of venous thromboembolic disease. Results of a French Multicentre Registry. Eur Heart J 1997;18:685–691.



16. Dentali F, Ageno W, Rancan E, et al. Seasonal and monthly variability in the incidence of venous thromboembolism. A systematic review and a meta-analysis of the literature. Thromb Haemost 2011;106:439–447.



17. Stein PD, Kayali F, Olson RE. Analysis of occurrence of venous thromboembolic disease in the four seasons. Am J Cardiol 2004;93:511–513.


18. Ribeiro DD, Bucciarelli P, Braekkan SK, et al. Seasonal variation of venous thrombosis: a consecutive case series within studies from Leiden, Milan and Tromso. J Thromb Haemost 2012;10:1704–1707.


19. Ro A, Hara M, Takada A. The factor V Leiden mutation and the prothrombin G20210A mutation was not found in Japanese patients with pulmonary thromboembolism. Thromb Haemost 1999;82:1769.

20. Choi HS, Choi CW, Kim HM, Park HW. Venous thromboembolism in pediatric patients: a single institution experience in Korea. Blood Res 2016;51:164–170.



21. Jang MJ, Kim HJ, Bang SM, et al. Seasonal variation in the occurrence of venous thromboembolism: a report from the Korean Venous Thromboembolism Working Party. Thromb Res 2012;130:e199.


22. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci 2017;32:718–728.



23. Hong J, Lee JH, Yhim HY, et al. Incidence of venous thromboembolism in Korea from 2009 to 2013. PLoS One 2019;13:e0191897.

24. Woodhouse PR, Khaw KT, Plummer M, Foley A, Meade TW. Seasonal variations of plasma fibrinogen and factor VII activity in the elderly: winter infections and death from cardiovascular disease. Lancet 1994;343:435–439.


25. Arntz HR, Willich SN, Schreiber C, Bruggemann T, Stern R, Schultheiss HP. Diurnal, weekly and seasonal variation of sudden death. Population-based analysis of 24,061 consecutive cases. Eur Heart J 2000;21:315–320.



26. Gallerani M, Portaluppi F, Maida G, et al. Circadian and circannual rhythmicity in the occurrence of subarachnoid hemorrhage. Stroke 1996;27:1793–1797.


27. Mehta RH, Manfredini R, Hassan F, et al. Chronobiological patterns of acute aortic dissection. Circulation 2002;106:1110–1115.


28. Spencer FA, Goldberg RJ, Becker RC, Gore JM. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol 1998;31:1226–1233.


29. Manfredini R, Boari B, Salmi R, Gallerani M. Seasonal variation of venous thromboembolic disease. Am J Cardiol 2004;94:276.

30. Gallerani M, Boari B, Smolensky MH, et al. Seasonal variation in occurrence of pulmonary embolism: analysis of the database of the Emilia-Romagna region, Italy. Chronobiol Int 2007;24:143–160.


31. Keatinge WR, Coleshaw SR, Cotter F, Mattock M, Murphy M, Chelliah R. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. Br Med J (Clin Res Ed) 1984;289:1405–1408.



32. Carder M, McNamee R, Beverland I, et al. The lagged effect of cold temperature and wind chill on cardiorespiratory mortality in Scotland. Occup Environ Med 2005;62:702–710.



33. Tseng CM, Chen YT, Ou SM, et al. The effect of cold temperature on increased exacerbation of chronic obstructive pulmonary disease: a nationwide study. PLoS One 2013;8:e57066.



34. Danai PA, Sinha S, Moss M, Haber MJ, Martin GS. Seasonal variation in the epidemiology of sepsis. Crit Care Med 2007;35:410–415.


35. Latha KM, Badarinath KV. Seasonal variations of PM10 and PM2.5 particles loading over tropical urban environment. Int J Environ Health Res 2005;15:63–68.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



