 |
 |
| Korean J Intern Med > Volume 35(3); 2020 > Article |
|
Abstract
Background/Aims
The eradication failure rate of standard triple therapy (proton pump inhibitor, clarithromycin, and amoxicillin) for Helicobacter pylori infection has increased owing to antibiotic resistance in Korea. We assessed whether Saccharomyces boulardii probiotic or broccoli sprout extract sulforaphane supplementation could increase the H. pylori eradication rate and/or reduce antibiotic-associated adverse events.
Methods
A total of 217 patients with H. pylori-positive chronic gastritis or peptic ulcer disease were recruited. Clarithromycin resistance was assessed in all patients by testing for A2142G and A2143G point mutations in H. pylori 23S rRNA using a dual-priming polymerase chain reaction (PCR) oligonucleotide. Thirty-four patients (17.3%) were clarithromycin-resistant and were excluded from the study. Finally, 183 patients with infections not resistant to clarithromycin were randomly assigned to triple therapy only (group A, n = 61), triple therapy plus probiotics (group B, n = 61), or triple therapy plus sulforaphane (group C, n = 61) groups. CYP2C19 polymorphisms were examined at position G681A of exon 5 and G636A of exon 4 by PCR with restriction fragment length polymorphism (PCR-RFLP) analysis. H. pylori eradication was assessed by 13C-urea breath test 4 weeks after treatment completion.
Results
The eradication rates were similar among the groups both in the intention-to-treat (A = 85.2%, B = 89.6%, and C = 81.6%) and per-protocol (A = 89.2%, B = 86.8%, and C = 96.3%) analyses. The frequencies of overall adverse events in the groups also did not differ (A vs. B: p = 0.574; A vs. C: p = 1.000).
Helicobacter pylori infection affects more than 50% of the worldŌĆÖs human population and is associated with gastritis, peptic ulcer disease, and gastric cancer [1]. In Korea, the H. pylori infection rate in adults is approximately 60% and gastric cancer is the second most frequently diagnosed malignancy [2,3]. Successful eradication of H. pylori could be beneficial in alleviating H. pylori-related gastroduodenal diseases and reduce the risk of gastric cancer in countries like Korea where the prevalence of both H. pylori infection and gastric cancer are high. Until now, the triple therapy combining proton pump inhibitor (PPI) with two antibiotics (clarithromycin and amoxicillin) has been the standard first-line treatment for H. pylori eradication. Nonetheless, the eradication rate of this regimen has decreased to < 80% [4-7].
The primary factor associated with eradication failure is clarithromycin resistance. In Korea, the clarithromycin resistance rate gradually increased from 17.2% to 23.7% during 2003 to 2008 [8]. The second factor related to eradication failure is increased rates of antibiotic-associated adverse events, which could result in poor patient compliance [9]. In areas with high clarithromycin resistance, bismuth-containing quadruple or non-bismuth quadruple therapies are recommended. Additionally, alternative treatment strategies that increase the eradication rate and reduce adverse events should be developed.
Probiotics supplementation with the standard triple therapy might be a candidate to satisfy this purpose. Administration of probiotics have reportedly improved H. pylori eradication rates and reduced adverse events associated with the triple therapy [10-12]. However, the inhibitory effect of probiotics on H. pylori infection remains controversial. Recently, two large meta-analyses showed that Saccharomyces boulardii reduced overall adverse events and increased eradication rates [13]. Sulforaphane extracted from broccoli is another supplementary candidate for H. pylori eradication therapy. Broccoli sprout extract containing sulforaphane (BSES) exhibits cellular anti-oxidative, anti-inflammatory, and anti-cancer effects [14-16]. Sulforaphane is a potent bacteriostatic agent against H. pylori strains and also exhibits bactericidal effects in human epithelial cells [17].
Although no statistically significant effects have been demonstrated in the previous study, we have identified a tendency to show positive results in some urea breath test (UBT) results; further studies have been presented on the effects of using the combination with standard triple therapy [18]. This study excluded patients with cultures resistant to clarithromycin and registered only those subjects with infections not resistant to clarithromycin. Based on our literature review, to our knowledge, this is the first study designed to eliminate clarithromycin resistance as the affecting bias of H. pylori eradication failure. We aimed to determine whether S. boulardii probiotics or sulforaphane supplementation could increase the H. pylori eradication rate and/or reduce antibiotic-associated adverse events in a Korean population.
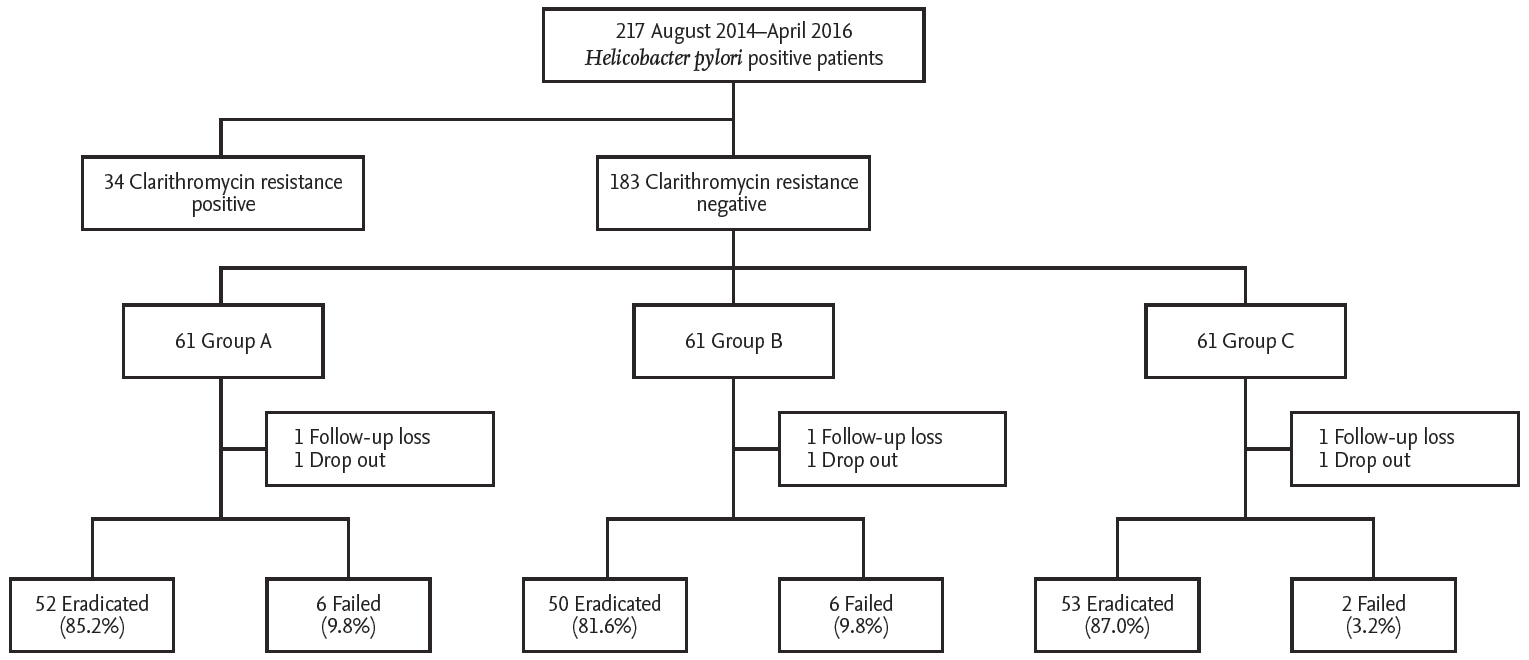
All patients with H. pylori-positive chronic gastritis or peptic ulcer disease were recruited at the Kyung Hee University Hospital in Seoul, Korea, from August 2014 to April 2016. All participants were diagnosed with H. pylori infection using rapid urease or C13-UBTs. Patients with gastric cancer, previous gastrectomy, and severe underlying systemic diseases, or those who had received antibiotics or PPI within the previous month were excluded. Data including age, gender, cigarette smoking, alcohol intake, salty food consumption, family history of gastric cancer, body mass index (BMI), hypertension, and diabetes were collected. All patients underwent clarithromycin resistance testing and subjects with clarithromycin-resistant infections were excluded from the study. Finally, subjects were registered and a computer program was used to randomly assigned the subjects into three treatment groups: triple therapy only (group A), triple therapy plus probiotics (group B), and triple therapy plus sulforaphane (group C) (Fig. 1). Group A received 40 mg of pantoprazole, 1 g of amoxicillin, and 500 mg of clarithromycin twice daily for 7 days. Groups B and C received the same PPI-based triple therapy for 7 days. Additionally, one capsule with either probiotics or sulforaphane was provided three times daily for 4 weeks to patients in groups B and C, respectively. All patients provided written informed consent prior to enrolment. This study was approved by the Institutional Review Board of the Kyung Hee University Hospital (KMC IRB: 1427-01) and registered with ClinicalTrials. gov (NCT03220542). All authors had access to the study data and reviewed and approved the final manuscript.
S. boulardii capsules (3 ├Ś 1010 colony-forming units/g, Bioflor, Kuhnil Pharm., Seoul, Korea) were administered as the probiotic. BSES capsules containing 250 mg standardized broccoli sprout, yielding 1,000 ╬╝g of sulforaphane (Oregon Health, Phoenix, AZ, USA), were administered. The doses from broccoli and probiotics were determined to meet the doses presented in several studies, including previous studies of us [18].
C13-UBTs were performed 4 weeks after the last dose of the study medication. One week after completing treatment, information on adverse events and compliance was collected from the outpatient department through telephone interviews. Compliance was considered satisfactory when patients had taken > 85% of the study medications.
Mutation of the 23S rRNA results in increased antibiotic resistance [19]. Clarithromycin resistance in H. pylori in the present study was determined by evaluating for the presence of the A2142G or A2143G mutations in 23S rRNA. H. pylori genomic DNA was extracted from two biopsy specimens from the antrum and body collected during endoscopy. The DNA was amplified using the Seeplex home-brew primer mix from the Seeplex ClaR-H. pylori polymerase chain reaction (PCR) kit (Seegene Inc., Seoul, Korea) that was developed using a dual-priming oligonucleotide system to detect the A2142G or A2143G mutations.
The genotyping of exons 4 and 5 in CYP2C19 was conducted by PCR with restriction fragment length polymorphism (PCR-RFLP). The polymorphism of CYP2C19 was detected as previously described [20]. The patients were classified into three groups according to CYP2C19 genotype: rapid metabolizers (RM) had exon 5, wildtype/exon 4, wild-type; intermediate metabolizers (IM) had exon 5, wild-type/exon 4, heterozygote or exon 5, heterozygote/exon 4, wild-type; and poor metabolizers (PM) had both mutated alleles.
Eradication was evaluated using intention-to-treat (ITT) and per-protocol (PP) analyses. Continuous variables are expressed as means ┬▒ standard deviation, and categorical variables as percentages. StudentŌĆÖs t test, chi-square test, and two-factor analysis of variation (ANOVA) were used. PASW Statistics for Windows version 22.0 (IBM Co., Armonk, NY, USA) was used for all statistical analyses. All statistical tests were two-tailed and p values of < 0.05 were assumed to indicate statistical significance.
A total of 217 patients with H. pylori-positive chronic gastritis or peptic ulcer disease were recruited during study periods. Of these, 34 subjects with clarithromycin-resistant infections were excluded from the study. The rate of clarithromycin resistance in this study was 17.3% (34/217 cases). Two cases were dropped. Finally, 183 patients with clarithromycin-sensitive infections were randomly assigned the subjects into three treatment groups: group A (n = 61), B (n = 61), and C (n = 61). The clinical parameters such as age, gender, cigarette smoking, alcohol intake, family history of gastric cancer, salty food consumption, and BMI, were similar among the three groups. Endoscopic finding and CYP2C19 genotype did not differ significantly between the three groups (Table 1).
The eradication rates according to ITT and PP analyses were 85.2% and 89.6% in group A, respectively. ITT and PP analyses showed 81.9% and 89.2% eradication rates in group B and 86.8% and 96.3% in group C, respectively. The eradication rates according to ITT and PP analyses were not significantly different across the groups based on two-group comparison tests (ITT analysis: A vs. B, p = 0.744; A vs. C, p = 1.000) (PP analysis: A vs. B, p = 0.313; A vs. C, p = 0.273). The eradication rate in group C was slightly higher compared to group A. However, the difference between the two groups (A vs. C) was not statistically significant (Table 2).
In order of frequency, the adverse events included taste disturbance, diarrhea, headache, epigastric pain, nausea, and urticaria. The frequency of overall adverse events in the three groups was not significantly different (A vs. B, p = 0.574; A vs. C, p = 1.000). The frequencies of gastrointestinal disturbances such as diarrhea, epigastric pain, and nausea were lower in group B compared to group A, but the difference was not statistically significant (p = 0.339) (Table 3).
Analysis of CYP2C19 genotypes revealed the RM genotype in 16.4%, 24.6%, and 21.3% of patients in groups A, B, and C, respectively. The frequency of the RM genotype did not differ significantly across the three groups as per the results of the multiple group comparison test (p = 0.532) (Table 1).
The Maastricht V/Florence Consensus Report stated that PPI-clarithromycin-amoxicillin triple therapy without prior susceptibility test should be reconsidered in regions with a clarithromycin resistance rate above 15% [21]. Based on our literature review, ours is the first study to exclude patients resistant to clarithromycin, which minimized the most significant bias associated with eradication failure. In our study, 17.3% of the patient infections were clarithromycin-resistant. In another recent Korean study, the clarithromycin resistance rate was > 20% [22]. This difference may be attributable to different tests used for assessing clarithromycin resistance as well as our study including a large number of relatively heathy subjects. Nonetheless, considering that the clarithromycin resistance rate was > 15%, alternative regimens are needed for first-line treatment in Korea. Bismuth quadruple (PPI, bismuth, tetracycline, and metronidazole) or concomitant non-bismuth quadruple (PPI, amoxicillin, clarithromycin, and metronidazole) therapies are recommended as first-line treatments [21]. However, these therapies are associated with poor patient compliance and increased adverse events owing to antibiotic overdose. Although not highly evident, supplemental therapy with probiotics or sulforaphane could increase eradication rates and decrease antibiotic-associated adverse events.
A meta-analysis of 10 clinical trials on the efficacy of probiotics in H. pylori treatment showed that Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparations increased the overall eradication rate and decreased the overall adverse events [23]. However, another meta-analysis of 33 randomized controlled trials (RCTs) could confirm this finding for only four individual strains (Lactobacillus acidophilus, L. casei, L. gasseri, and Bifidobacterium infantis) [24]. This study also performed sub-group analysis according to the effectiveness of eradication regimens. In that study, the less effective the antibiotic therapies, the more useful the probiotic supplementation was: for eradication rates < 60%, the pooled risk ratio (RR) was 1.28 (1.12 to 1.45), for eradication rates of 60% to 69%, the pooled RR was 1.18 (1.10 to 1.27); for eradication rates of 70% to 79%, the pooled RR was (1.11; 1.06 to 1.17); and supplementation had no effect for eradication rates > 80% (pooled RR, 1.01; 0.96 to 1.77). The authors also reported a significant difference between the probiotics supplement and triple therapy only groups in the overall incidence of side effects (RR, 0.735; 95% confidence interval, 0.598 to 0.902) [24]. However, the result of side effects was only confirmed for non-blinded trials.
One double-blind RCT showed that Bacillus clausii significantly reduced gastrointestinal adverse events such as nausea, epigastric pain, and diarrhea but insignificantly increased eradication rates [25]. Recently, a meta-analysis of 11 RCTs showed that S. boulardii significantly increased eradication rates but that the rates were still below the desired level of success [13]. Probiotics reduce antibiotic-associated adverse events such as nausea, diarrhea, and abdominal pain, thus helping the completion of eradication therapy. Therefore, probiotics can improve the H. pylori eradication rate by reducing adverse events rather than by directly eradicating H. pylori. The proposed mechanism of probiotics is to counteract the harmful effects of antibiotics on gut microbiota. However, the opposite conclusions were drawn regarding side effects in three studies reporting no significant reduction in overall side effects with probiotics use [26-28].
Our study showed that probiotic supplementation did not significantly increase the eradication rate. This may be because of several factors. Firstly, the number of subjects was small. Secondly, the eradication rate was > 80% and so likely did not contribute an additive advantage, similar to that reported by Dang et al. [24]. Overall, the rate of adverse events was lower in the probiotics supplement group B (triple therapy plus probiotics group) compared to group A (standard triple therapy only group). However, the difference was not statistically significant.
Sulforaphane is a molecule within the isothiocyanate group of organosulfur compounds. It can be obtained from cruciferous vegetables such as broccoli, Brussels sprouts, and cabbages. Sulforaphane enhances the protection and repair of the gastric mucosa against oxidative stress in vitro and has been shown to have anti-inflammatory effects on H. pylori-infected gastric mucosae in mice and human subjects [29]. Sulforaphane has a potent bacteriostatic and bactericidal component against H. pylori in vitro [17]. Furthermore, it is highly effective against a large number of clinical isolates of H. pylori, which are highly resistant to conventional antibiotics [17]. Recently, sulforaphane was reported to reduce colonization and attenuate gastritis in H. pylori-infected mice and humans [30]. However, a Japanese epidemiological investigation of the relationship between the prevalence of H. pylori-induced chronic atrophic gastritis (CAG) and broccoli consumption found no association between frequent broccoli consumption and a low prevalence of CAG. In addition, our previous study did not show the inhibitory effect of sulforaphane on H. pylori colonization density in the gastric mucosa of patients with H. pylori-positive gastritis [18].
In the present study, the eradication rate in the sulforaphane supplementation group was slightly higher than that in group A (standard triple therapy only group), but the difference was not statistically significant. The overall rate of adverse events was also similar to group A.
Our study has some limitations. First, we included relatively small number of subjects in each group. However, this study was a single center study, and it had limitation to recruit enough patients during study period. Instead, during the study period, all patients who agreed to participate were enrolled by our study. In our study, probiotic supplementation and sulforaphane did not significantly increase the eradication rate nor decrease the adverse events. Nevertheless, the eradication rate in sulforaphane group was slightly higher than that in only triple therapy group. A further study including large number of subjects is needed in the future. Second, we used only S. boulardii capsules as the probiotic. The probiotics most commonly used include Lactobacillus spp., Bacillus spp., and yeast such as S. boulardii. The choice of the S. boulardii was determined by the effectiveness as well as being the most commonly used in most studies.
In conclusion, we found that supplement therapy with probiotics or sulforaphane for H. pylori eradication was ineffective at increasing eradication rates and decreasing adverse events.
1. Probiotic or sulforaphane supplementation with triple therapy for Helicobacter pylori infection did not significantly increase the eradication rate nor reduced the adverse events.
2. An appropriate treatment strategy and new drawn up guidelines on treatment of H. pylori are necessary for the effective and safety eradication of H. pylori.
Acknowledgments
We appreciate the excellent support of our nursing team at the Kyung Hee University Hospital-Digestive Endoscopy Center.
Table┬Ā1.
Baseline characteristics of the study population
Table┬Ā2.
Helicobacter pylori eradication rates among the three treatment groups
| Variable |
Eradication rate |
p valuea | p valueb | ||
|---|---|---|---|---|---|
| Group A (n = 61) | Group B (n = 61) | Group C (n = 61) | |||
| Eradicated | 52 | 50 | 53 | ||
| Failed | 6 | 6 | 2 | ||
| Follow-up loss | 1 | 5 | 6 | ||
| Dropped out | 2 | 0 | 0 | ||
| Poor compliance | 0 | 0 | 0 | ||
| ITT analysis | 52/61 (85.2) | 50/61 (81.9) | 53/61 (86.8) | 0.744 | 1.000 |
| PP analysis | 52/58 (89.6) | 50/56 (89.2) | 53/55 (96.3) | 0.313 | 0.273 |
Table┬Ā3.
Adverse events in the three treatment groups
| Variable | Group A (n = 61) | Group B (n = 61) | Group C (n = 61) | p valuea | p valueb |
|---|---|---|---|---|---|
| Taste disturbance | 30 | 29 | 28 | 1.000 | 1.000 |
| Diarrhea | 9 | 5 | 8 | 0.396 | 1.000 |
| Headache | 5 | 2 | 6 | 0.440 | 0.758 |
| Epigastric pain | 4 | 3 | 3 | 1.000 | 1.000 |
| Nausea | 3 | 2 | 1 | 1.000 | 0.619 |
| Urticaria | 3 | 1 | 1 | 0.619 | 0.619 |
| GI disturbancec | 13 | 8 | 12 | 0.339 | 0.482 |
| Total | 36 | 31 | 34 | 0.574 | 1.000 |
REFERENCES
2. Kim YS, Baik GH. Epidemiology of Helicobacter pylori infection in Korea. Korean J Helicobacter Up Gastrointest Res 2011;11:1ŌĆō6.


3. Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat 2012;44:11ŌĆō24.




4. Cho DK, Park SY, Kee WJ, et al. The trend of eradication rate of Helicobacter pylori infection and clinical factors that affect the eradication of first-line therapy. Korean J Gastroenterol 2010;55:368ŌĆō375.


5. Choi YS, Cheon JH, Lee JY, et al. The trend of eradication rates of first-line triple therapy for Helicobacter pylori infection: single center experience for recent eight years. Korean J Gastroenterol 2006;48:156ŌĆō161.

6. Kim BJ, Kim HS, Song HJ, et al. Online registry for nationwide database of current trend of Helicobacter pylori eradication in Korea: interim analysis. J Korean Med Sci 2016;31:1246ŌĆō1253.



8. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter 2013;18:206ŌĆō214.


9. Bell GD, Powell K, Burridge SM, et al. Experience with ŌĆśtripleŌĆÖ anti-Helicobacter pylori eradication therapy: side effects and the importance of testing the pre-treatment bacterial isolate for metronidazole resistance. Aliment Pharmacol Ther 1992;6:427ŌĆō435.


10. Tong JL, Ran ZH, Shen J, Zhang CX, Xiao SD. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2007;25:155ŌĆō168.


11. Song MJ, Park DI, Park JH, et al. The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter 2010;15:206ŌĆō213.


12. Bekar O, Yilmaz Y, Gulten M. Kefir improves the efficacy and tolerability of triple therapy in eradicating Helicobacter pylori. J Med Food 2011;14:344ŌĆō347.


13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther 2010;32:1069ŌĆō1079.


14. Guerrero-Beltran CE, Calderon-Oliver M, Pedraza-Chaverri J, Chirino YI. Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicol Pathol 2012;64:503ŌĆō508.


15. Brandenburg LO, Kipp M, Lucius R, Pufe T, Wruck CJ. Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflamm Res 2010;59:443ŌĆō450.



16. Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A 1992;89:2399ŌĆō2403.



17. Fahey JW, Haristoy X, Dolan PM, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a] pyrene-induced stomach tumors. Proc Natl Acad Sci U S A 2002;99:7610ŌĆō7615.



18. Chang YW, Jang JY, Kim YH, Kim JW, Shim JJ. The effects of broccoli sprout extract containing sulforaphane on lipid peroxidation and Helicobacter pylori Infection in the gastric mucosa. Gut Liver 2015;9:486ŌĆō493.



19. Occhialini A, Urdaci M, Doucet-Populaire F, Bebear CM, Lamouliatte H, Megraud F. Macrolide resistance in Helicobacter pylori: rapid detection of point mutations and assays of macrolide binding to ribosomes. Antimicrob Agents Chemother 1997;41:2724ŌĆō2728.



20. Andersson T, Regardh CG, Lou YC, Zhang Y, Dahl ML, Bertilsson L. Polymorphic hydroxylation of S-mephenytoin and omeprazole metabolism in Caucasian and Chinese subjects. Pharmacogenetics 1992;2:25ŌĆō31.


21. Malfertheiner P, Megraud F, OŌĆÖMorain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017;66:6ŌĆō30.


22. Lee JY, Kim N, Kim MS, et al. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci 2014;59:1235ŌĆō1243.



23. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol 2013;47:25ŌĆō32.


24. Dang Y, Reinhardt JD, Zhou X, Zhang G. The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: a meta-analysis. PLoS One 2014;9:e111030.



25. Nista EC, Candelli M, Cremonini F, et al. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-blind, placebo controlled trial. Aliment Pharmacol Ther 2004;20:1181ŌĆō1188.


26. Zou J, Dong J, Yu X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter 2009;14:97ŌĆō107.


27. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol 2009;21:45ŌĆō53.


28. Zheng X, Lyu L, Mei Z. Lactobacillus-containing probiotic supplementation increases Helicobacter pylori eradication rate: evidence from a meta-analysis. Rev Esp Enferm Dig 2013;105:445ŌĆō453.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



