 |
 |
| Korean J Intern Med > Volume 35(3); 2020 > Article |
|
Abstract
Figure 1.
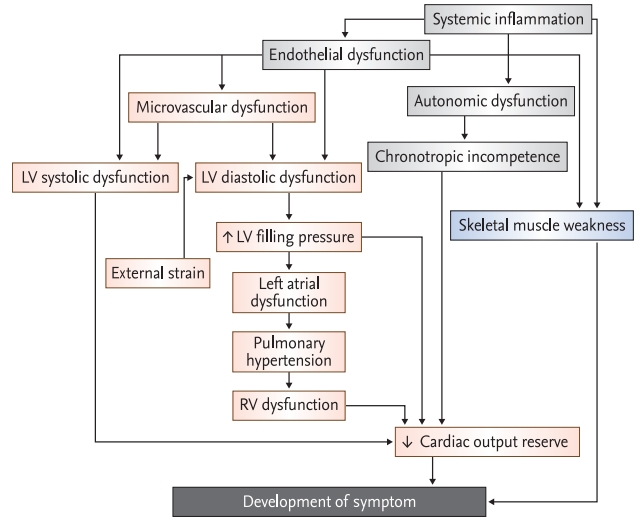
Figure 2.
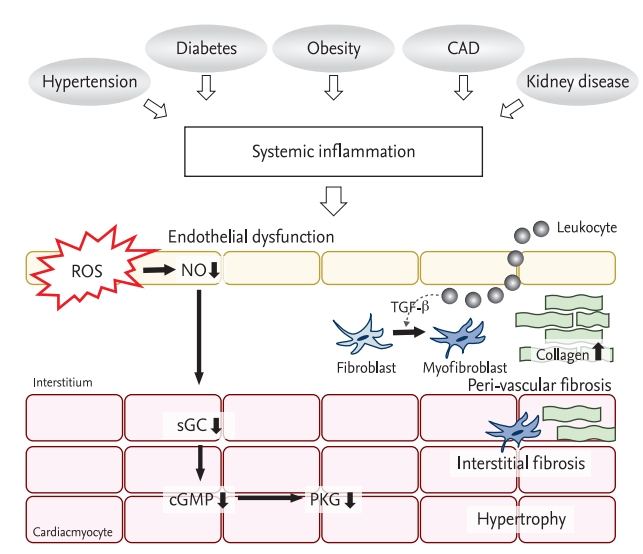
Table 1.
|
Current guideline of HFpEF |
|||
|---|---|---|---|
| 2016 KSHF guideline [7] | 2016 ESC guideline [8] | 2013 AHA guideline [9] | |
| Clinical manifestation | Symptoms and signs of HF | Symptoms and signs of HF | Symptoms and signs of HF |
| LVEF | ≥ 50% | ≥ 50% | ≥ 50% |
| Natriuretic peptides | BNP ≥ 35 pg/mL or NT-proBNP ≥ 125 pg/mL | BNP > 35 pg/mL or NT-proBNP > 125 pg/mL | |
| Imaging | Abnormal LVDD | Relevant structural heart disease (LVH and/or LAE) | Abnormal LVDD |
| LVDD | |||
| -LAVI > 34 mL/m2 | |||
| -LVMI ≥ 115/95 g/m2 (M/W) | |||
| -E/e’ ≥ 13 | |||
| -Mean e’< 9 cm/sec | |||
HFpEF, heart failure with preserved ejection fraction; KSHF, Korean Society of Heart Failure; ESC, European Society of Cardiology; AHA, American Heart Association; HF, heart failure; LVEF, left ventricular ejection fraction; BNP, B-type natriuretic peptide; NT-proBNP, N-terminal pro-B type natriuretic peptide; LVDD, left ventricular diastolic dysfunction; LVH, left ventricular hypertrophy; LAE, left atrial enlargement; LAVI, left atrial volume index; LVMI, left ventricular mass index; M/W, men/women.
Table 2.
| Clinical variable | H2FpEF score [14] | Point | |
|---|---|---|---|
| H2 | Heavy | BMI > 30 kg/m2 | 2 |
| Hypertension | Antihypertensive medication ≥ 2 | 1 | |
| F | Atrial fibrillation | Paroxysmal or persistent | 3 |
| P | Pulmonary hypertension | Doppler echocardiographic estimated PASP > 35 mmHg | 1 |
| E | Elder | Age > 60 years | 1 |
| F | Filling pressure | Doppler echocardiographic E/e’ > 9 | 1 |
| Sum | 0–9 | ||
Table 3.
|
HFA-PEFF score [15] |
|||||
|---|---|---|---|---|---|
|
Major |
Minor |
||||
| Value | Point | Value | Point | ||
| Functional | Septal e’ < 7 cm/sec or lateral e’ < 10 cm/sec | 2 | Avergaed E/e’ 9–14 | 1 | |
| or | or | ||||
| Averaged E/e’ ≥ 15 | GLS < 16% | ||||
| or | |||||
| TR Vmax > 2.8 m/sec (PASP > 35 mmHg) | |||||
| Morphological | LAVI > 34 mL/m2 | 2 | LAVI 29–34 mL/m2 | 1 | |
| or | or | ||||
| LVMI ≥ 149/122 g/m2 (M/W) ± RWT > 0.42 | LVMI ≥ 115/95 m2 (M/W) | ||||
| or | |||||
| RWT > 0.42 | |||||
| or | |||||
| LV wall thickness ≥ 12 mm | |||||
| Biomarker | (SR) | NT-proBNP > 220 pg/mL | 2 | NT-proBNP > 660 pg/mL | 1 |
| or | or | ||||
| BNP > 80 pg/mL | BNP > 240 pg/mL | ||||
| (AF) | NT-proBNP 125–220 pg/mL | 2 | NT-proBNP 365–660 pg/mL | 1 | |
| or | or | ||||
| BNP 35–80 pg/mL | BNP 105–240 pg/mL | ||||
≥ 5 points: heart failure with preserved ejection fraction; 2–4 points: exercise stress test or invasive hymodynamic measurement.
HFA-PEFF, heart Failure Association-PEFF; TR, tricuspid regurgitation; PASP, pulmonary artery systolic pressure; GLS, global longitudinal strain; LAVI, left atrial volume index; LVMI, left ventricular mass index; M/W, men/women; RWT, regional wall thickness; LV, left ventricular; SR, sinus rhythm; NT-proBNP, N-terminal pro-B type natriuretic peptide; BNP, B type natriuretic peptide; AF, atrial fibrillation.
Table 4.
| Trials, year (no. of patients) | Interventions | Inclusion criteria | Characteristics of study population | Primary endpoint | Trial result | |
|---|---|---|---|---|---|---|
| Renin-angiotensin-aldosterone system | ||||||
| CHARM-preserved 2003 (n = 2,023) [58] | Candesartan | NYHA II–IV HF with prior cardiac hospitalization | Mean age: 67 ± 11 years, 40% female 65% hypertension, 28% diabetes | CV death or HF hospitalization | No benefit for primary endpoint | |
| Mean LVEF: 54% ± 9.4% | Reduction of HF hospitalization | |||||
| LVEF ≥ 40% | Median FU: 36.6 months | |||||
| PEP-CHF 2006 (n = 850) [59] | Perindopril | Age ≥ 70 years | Mean age: 76 ± 5 years, 55% female 79% hypertension, 20% diabetes | All cause mortality or HF hospitalization | No benefit for primary endpoint | |
| Diastolic heart failure | Mean LVEF: 64% | Reduction of HF hospitalization | ||||
| Median FU: 2.1 years | Improvement of symptom and exercise capacity | |||||
| I-PRESERVED 2008 (n = 4,128) [60] | Irbesartan | Age ≥ 60 years | Mean age: 72 ± 7 years, 60% female 88% hypertension, 27% diabetes | Death from any cause or hospitalization for CV cause | No benefit for primary endpoint | |
| NYHA II–IV | Mean LVEF: 60% ± 9% | No improvement of QOL | ||||
| LVEF ≥ 45% | Mean FU: 49.5 months | |||||
| TOPCAT 2014 (n = 3,445) [63] | Spironolactone | HF with history of HF hospitalization within 12month and elevated BNP within 60 days | Median age: 69 years, 52% female 91% hypertension, 32% diabetes | CV death or cardiac arrest or HF hospitalization | No benefit for primary endpoint | |
| Median LVEF: 56% | Reduction of HF hospitalization | |||||
| LVEF ≥ 45% | Mean FU: 3.3 years | |||||
| PARAGON-HF 2019 (n = 4,822) [66] | Sacubitrilvalsartan | NYHA II–IV | Mean age: 73 ± 8 years, 52% female 96% hypertension, 43% diabetes | HF hospitalization or CV death | No benefit for primary endpoint | |
| LVEF ≥ 45% | Mean LVEF: 60% ± 9% | Reduce primary endpoint in subgroup (patients wuth LVEF below median [≤ 57%] and women) | ||||
| Elevated NPs | Total FU time up to 57 months | |||||
| Beta-blocker | ||||||
| SENIORS 2009 (n = 752) [80] | Nebivolol | CHF history | Mean age: 76 ± 5 years, 51% female 78% hypertension, 24% diabetes | All cause mortality or CV hospitalization | No benefit for primary endpoint | |
| HF hospitalization within 12month | Mean LVEF: 49% ± 10% | |||||
| FU: 21 months | ||||||
| J-DHF 2013 (n = 245) [81] | Carvedilol | LVEF ≥ 40% | Mean age: 76 ± 5 years, 55% female 80% hypertension, 33% diabetes | CV death or HF hospi talization | No benefit for primary endpoint | |
| Mean LVEF: 63% ± 11% | Reduce primary endpoints in patients with higher dose | |||||
| Median FU: 3.2 years | ||||||
| Disease-modifying agents | ||||||
| NEAT-HFpEF 2015 (n = 110) [88] | Isosorbide mononitrate (organic nitrate) | LVEF ≥ 50% | Mean age: 69 ± 9 years, 49% female 88% hypertension, 43% diabetes | Daily activity level | Decreased activity and worsen QOL | |
| HF with objective evidence (≥ 1) | Mean LVEF: 62% ± 8% | |||||
| - HF hospitalization with congestion , increased LVEDP or PCWP, elevated NP, LVDD on ECHO | Drug intervention for 6 weeks | |||||
| INDIE-HFpEF 2018 (n = 105) [94] | Nebulized inorganic nitrate | Age ≥ 40 years | Mean age: 68 ± 9 years, 68% female 81% hypertension, 38% diabetes | Peak O2 consumption | No improvement in exercise capacity | |
| LVEF ≥ 50% | Mean LVEF: 61% ± 5% | |||||
| HF with objective evidence (≥ 1) | Drug intervention for 4 weeks | |||||
| - HF hospitalization with congestion, increased LVEDP or PCWP, elevated NP, LVDD on ECHO | ||||||
| DILATE-1 2014 (n = 21) [95] | Riociguat (sGC stimulator) | HFpEF with PH | Mean age: 68 ± 9 years, 68% female 81% hypertension, 44% diabetes | Peak decrease in mPAP | No significant effect on mPAP | |
| LVEF ≥ 50% | ||||||
| mPAP ≥ 25 mmHg | Mean LVEF: 62% ± 7% | |||||
| PAWP > 15 mmHg at rest | ||||||
| SOCRATES-PRESERVED 2017 (n = 477) [96] | Vericiguat (sGC stimulator) | NYHA II–IV | Mean age: 73 ± 10 years, 48% female 49% diabetes | Change of NT-proBNP and LAV | No signigicant change of NT-pro BNP and LAV | |
| LVEF ≥ 45% | Mean LVEF: 57% | |||||
| Elevated NPs | Drug intervention for 4 weeks | |||||
| History of HF hospitalization or IV diuretics within 4 weeks | ||||||
| RELAX 2016 (n = 216) [98] | Sildenafil | HFpEF with RVD and RV-RA coupling | Mean age: 68 ± 9 years, 68% female 81% hypertension, 38% diabetes | Peak oxygen uptake | No improvement of RV function, exercise capacity and ventilatory efficiency | |
| NYHA II–IV | ||||||
| LVEF ≥ 50% | Mean LVEF: 61% ± 5% | |||||
| Evidence of HF (≥ 1) | Drug intervention for 4 weeks | |||||
| - HF hospitalization, elevated LVFP and LAE | ||||||
| Non-pharmacological therapy | ||||||
| CHAMPION 2011 (n = 119) [101] | Wireless implantable hemodynamic monitoring | HF with NYHA III | Mean age: 66 ± 12 years, 40% female 82% hypertension, 58% diabetes | HF hospitalization | Reduction of HF hospitalization | |
| LVEF ≥ 40% | Drug intervention for 6 months | |||||
| REDUCE LAP-HF 2016 (n = 66) [103] | Interatrial shunt device | Age ≥ 40 years | Mean age: 69 ± 8 years, 65% female 81% hypertension, 33% diabetes | Successful device implantation | Safe | |
| LVEF ≥ 40% | Drug intervention for 6 months | Reduction of PCWP | Reduction of LAP during exercise during exercise | |||
| Exercise PCWP ≥ 25 mmHg, PCWP-RAP gradient ≥ 5 mmHg | ||||||
| REDUCE LAP-HF I 2018 (n = 94) [104] | Interatrial shunt device | NYHA III, IV | Mean age: 70 ± 9 years, 50% female 82% hypertension, 55% diabetes | Exercise PCWP | Reduction of PCWP during exercise | |
| LVEF ≥ 40% | ||||||
| Exercise PCWP ≥ 25 mmHg, PCWP-RAP gradient ≥ 5 mmHg | Drug intervention for 1 months | |||||
| Manage of comorbidities | ||||||
| Ex-DHF 2011 (n = 64) [115] | Endurance/resistance training | Age ≥ 45 years | Mean age: 65 ± 7 years, 56% female 86% hypertension, 14% diabetes | Peak VO2 | Improvement of exercise capacity and QOL | |
| NYHA II–III | ||||||
| LVEF ≥ 50% | Drug intervention for 3 months | |||||
| CV risk factor (overweight, diabetes, hypertension, hyperlipidemia, smoking) | ||||||
| SECRET-1 2016 (n = 200) [117] | Caloric restriction | HF with obesity | Mean age: 61 ± 5 years, 56% female 95% hypertension, 35% diabetes | Peak VO2 | Increased of peak oxygen uptake both caloric restriction and aerobic exercise training with addictive value | |
| Age ≥ 60 years | Disease-specific QOL | |||||
| Aerobic exercise training | BMI ≥ 30 | Intervention for 3 months | ||||
| LVEF ≥ 50% | ||||||
HFpEF, heart failure with preserved ejection fraction; CHARM, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity; NYHA, New York Heart Association; HF, heart failure; LVEF, left ventricular ejection fraction; FU, follow-up; CV, cardiovascular; PEP-CHF, Perindopril in Elderly people with Chronic Heart Failure; I-PRESERVED, Irbesartan in Heart Failure With Preserved Ejection Fraction; QOL, quality of life; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial; BNP, brain natriuretic peptide; PARAGON-HF, Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction; NP, natriuretic peptide; SENIORS, Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure; CHF, congestive heart failure; J-DHF, Japanese Diastolic Heart Failure Study; NEAT-HFpEF, Nitrate’s Effect on Activity Tolerance in Heart Failure with Preserved Ejection Fraction; LVEDP, left ventricular end diastolic pressure; PCWP, pulmonary capillary wedge pressure; LVDD, left ventricular diastolic dysfunction; ECHO, echocardiography; INDIE-HFpEF, Inorganic Nitrite Delivery to Improve Exercise Capacity in HFpEF; DILATE-1, Acute Hemodynamic Effects of Riociguat in Patients with Pulmonary Hypertension Associated with Diastolic HF; sGC, soluble guanylate cyclase; PH, pulmonary hypertension; mPAP, mean pulmonary artery pressure; PAWP, pulmonary arterial wedge pressure; SOCRATES-PRESERVED, Soluble guanylate Cyclase stimulatoR in heArT failurE patients with PRESERVED EF; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LAV, left atrial volume; RELAX, phosphodiesterase-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure; RVD, right ventricular dysfunction; RV-RA, right ventricular-right atrial; LVFP, left ventricular filling pressure; LAE, left atrial enlargement; CHAMPION, CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients; REDUCE LAP-HF, Reduce Elevated Left Atrial Pressure in Patients with Heart Failure; RAP, right atrial pressure; Ex-DHF, enhancement of physical activity in elderly patients with diastolic heart failure; VO2, oxygen uptake; SECRET-1, Study of the Effect of Caloric Restriction and Exercise Training in patients with heart failure and a normal ejection fraction-1; BMI, body mass index.
Table 5.
HFpEF, heart failure with preserved ejection fraction; SPIRRIT-HFPEF, Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure with Preserved Ejection Fraction ; LVEF, left ventricular ejection fraction; HF, heart failure; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SR, sinus rhythm; AF, atrial fibrillation; CV, cardiovascular; PARAGLIDE-HF, A Multicenter, Randomized, Double-blind, Double-dummy, Parallel Group, Active Controlled Study to Evaluate the Effect of Sacubitril/Valsartan (LCZ696) Versus Valsartan on Changes in NT-proBNP and Outcomes, Safety, and Tolerability in HFpEF Patients With Acute Decompensated Heart Failure (ADHF) Who Have Been Stabilized During Hospitalization and Initiated In-hospital or Within 30 Days Post-discharge; PRISTINE-HF, PRospectIve Study of Sacubitril/ValsarTan on MyocardIal OxygenatioN and Fibrosis in PatiEnts With Heart Failure and Preserved Ejection Fraction; NYHA, New York Heart Association; LAE, left atrial enlargement; LVH, left ventricular hypertrophy; OS-CMR, oxygenation sensitive cardiac magnetic resoance; KNO3CK OUT HFPEF, Effect of KNO3 Compared to KCl on Oxygen UpTake in Heart Failure With Preserved Ejection Fraction; VO2, oxygen uptake; CAPACITY-HFpEF, A Multicenter, Randomized, Double-blind, Placebo-controlled, Phase 2 Study Evaluating the Safety and Efficacy of Different Doses of IW-1973 Over 12 Weeks in Patients With Heart Failure With Preserved Ejection Fraction; sGC, soluble guanylate cyclase; BMI, body mass index; SATELLITE, Safety and Tolerability Study of AZD4831 in Patients With Heart Failure; PCWP, pulmonary capillary wedge pressure; DELIVER, Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure; DETERMINE-preserved, Dapagliflozin Effect on Exercise Capacity Using a 6-minute Walk Test in Patients With Heart Failure With Preserved Ejection Fraction; 6MWD, 6-minute walk distane; EMPEROR-Preserved, EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Preserved Ejection Fraction; FAIR-HFpEF, Effect of IV Iron in Patients With Heart Failure With Preserved Ejection Fraction; IV, intravenous; LVDD, left ventricular diastolic dysfunction; Hb, hemoglobin; 6MWT, 6-minute walk teast; INABLE-traing, Inorganic Nitrite to Amplify the Benefits and Tolerability of Exercise Training in Heart Failure With Preserved Ejection Fraction; RAPID-HF, Rate-Adaptive Atrial Pacing In Diastolic Heart Failure; CCM-HFpEF, Cardiac Contractility Modulation Therapy in Subjects With Heart Failure With Preserved Ejection Fraction; RHC, right heart catheterization; LAVI, left atrial volume index; LVEDVI, left ventricular end-diastolic volume index.
REFERENCES
-
METRICS

- Related articles
-
Breakthrough in heart failure with preserved ejection fraction: are we there yet?2016 January;31(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


