 |
 |
| Korean J Intern Med > Volume 22(4); 2007 > Article |
|
Abstract
Background
Weight loss is common in patients with chronic obstructive pulmonary disease (COPD). However, the mechanisms of this weight loss are still unclear.
Methods
Sixty male patients with stable COPD and 45 healthy male controls participated in this study. The COPD patients were divided into two groups, that is, the emphysema and chronic bronchitis groups, by the transfer coefficient of carbon monoxide. The body composition, resting energy expenditure (REE), plasma leptin levels and serum tumor necrosis factor-alpha (TNF-α) were measured in all the study participants. The difference and correlation of these parameters were investigated between the two groups.
Results
Emphysematous patients were characterized by a lower body mass index (BMI) and fat-mass (FM)
compared with the chronic bronchitis patients (p<0.001). The plasma leptin levels, as corrected for the FM, were not different between the COPD patients and healthy controls (78.3±30.9 pg/mL/kg vs. 70.9±17.3 pg/mL/kg, respectively), and the plasma leptin levels, as adjusted for the FM, were also not different between the two groups of COPD patients. In the chronic bronchitis patients, the plasma leptin concentration was correlated with the BMI (r=0.866, p<0.001) but it was not correlated with the BMI in the emphysema patients. The serum TNF-α levels were higher in the stable COPD patients than those in the controls, but there was no statistical difference (10.7±18.6 pg/mL vs. 7.2×3.5 pg/mL, respectively, p0.05). The leptin concentration was well correlated with the BMI and %FM in the patients with chronic bronchitis and the leptin concentration was only correlated with the %FM (r=0.450, p=0.027) in emphysema patients. There was no correlation between the plasma leptin concentration, as adjusted for the fat mass, and the activity of the TNF-α system.
Weight loss in patients with chronic obstructive pulmonary disease (COPD) is considered to be a natural course of the disease and it is understood to be a type of adaptation process to minimize oxygen consumption1, 2). However, the weight loss in COPD patients has been shown to be an independent risk factor for reducing the capacity to perform physical activity and to influence their death rate3-5), and so there is increasing interest on the mechanism of this weight loss.
The body composition of COPD patients varies according to the subtypes of disease. It has been reported that the reduction of body fat in patients with emphysema is more severe than that in those patients with chronic bronchitis6). Although the chronic bronchitis patients appeared to maintain their weight to some degree, the fat-free mass may actually be decreased.
Leptin is a product of obesity genes, and it plays an important role not only in energy metabolism, but also in glucose and lipid metabolism7-10). Leptin production is acutely increased in pathologic conditions such as infection or inflammation11), chronic renal insufficiency12) and sepsis13), which causes weight loss by inducing anorexia and hypermetabolism.
In animal studies, administration of inflammatory cytokines that induce anorexia, such as tumor necrosis factor-α (TNF-α) or interleukin-1, resulted in the up-regulation of leptin mRNA in adipose tissues and an in increase of the serum leptin concentration12, 14, 15). This finding suggests that leptin is not under a normal regulation mechanism and it is influenced by inflammatory mediators, and such a reaction may act as a mechanism of weight loss.
We evaluated the differences of body composition in stable patient with COPD and we also assessed the plasma leptin and TNF-α concentrations in clinically stable COPD patients. The aim of this study was to investigate the relationship between the plasma leptin concentration and the activity of the TNF-α system, including the soluble TNF receptors (sTNF-R55 and sTNF-R75), in those patients.
Sixty male patients with stable COPD were diagnosed according to the criteria of the American Thoracic Society16). Their irreversible chronic airflow obstruction was confirmed by performing body plethysmograpy. The patients had been clinically stable for at least 3 months prior to the study and they lacked clinical signs of exacerbation such as the increase of sputum production or purulence, and exacerbation of dyspnea. We exclude those patients who had conditions known to influence the leptin or TNF-α levels such as malignancy, infection, cardiac failure, recent surgery, endocrine disorders, collagen vascular disease and corticosteroid use.
Pulmonary function was measured with a compute-assisted plethysmography (Vmax 229, Sensor Medics, Yorba Linda, California, U.S.A.). The highest value from at least three maneuvers was used and the lung function values were expressed as a percentage of the normal predicted value.
Emphysema was differentiated from chronic bronchitis by the clinical findings and the diffusion capacity, in other words, the kco (transfer coefficient of carbon monoxide, DLco/VA) was applied17). Among the patients with findings on simple chest X-ray that both diaphragms were flat or with the depressed downward pattern, the posterior space of the sternum was dilated and there was reduced contrast of the local or systemic bronchitis-blood vessels, those cases with a kco lower than 60 were classified as having emphysema, and among the patients with increased contrast in the bronchitis-blood vessels seen on chest X-ray, the cases with a kco higher than 80 were classified as having chronic bronchitis
Among the 60 male subjects, there were 27 chronic bronchitis patients and 24 emphysema patients. 9 patients could not be classified as having emphysema or chronic bronchitis because their kco values were 60~79.
Forty five age-matched health male volunteers were studied as control subjects. The control subjects had no medical illness and they had normal physical examinations. Their complete blood cell counts and blood chemistries were normal. They showed no symptoms or signs of infection at the time of this study.
Body weight and height were measured for all subjects after an overnight fast. Body composition analysis was performed by applying the bioelectrical impedance method with using an instrument and software made by the Medigate Cooperation (BCA-100, Seoul, Korea). The body mass index (BMI), fat mass (FM), and fat-free mass (FFM) were then calculated.
Resting energy expenditure (REE) was measured by an open-circuit indirect calorimetry system with using a ventilated hood (Sensor Medics Vmax 229, Yorba Linda, California, U.S.A.). The measurements of REE were performed at 6 a.m. with the patients awake and in a comfortable supine position. The patients were in a fasting state from 9 p.m. the previous day and they rested about 30 minutes prior to the measurement. The head of the patient was covered with a canopy (Beckman Canopy System, Anheim, California, U.S.A.) and the leakage of air to outside of the canopy was prevented. The REE was only measured from the steady state. Steady states were defined as intervals of time (a minimum of 5min) during which the average oxygen consumption and carbon dioxide production changed by less than 10% and 5%, respectively. The REE was calculated from the oxygen consumption and carbon dioxide production with using Weir's formula. The expected value of the REE was obtained by the HarrisBenedict equation.
For all subjects, blood was drawn between 8:00 and 9:00 a.m. by venipuncture after fasting since 9:00 p.m. the previous night. Both the serum and plasma were separated from the blood cells by centrifugation at 1,000 rpm for 5 min. All the samples were stored at -70℃ until they were analyzed.
The serum leptin levels were measured by a highly sensitive human leptin RIA kit (Linco Res, Inc., St. Louis, MO, U.S.A.).
The TNF-α and its receptor (TNF-R55 and TNF-R75) levels were determined in the serum with using a duplicate enzymelinked immunosorbent assay (ELISA) kit (Quantikine; R & D system, Minneapolis, MN, U.S.A.). The color generated was determined with using a spectrophotometric microtiter plate reader (Model 450; Bio-Rad, Richmond, California, U.S.A.) by measuring the optical density at 490 nm and 450 nm for the TNF-α and TNF-receptors (sTNF-R55 and sTNF-R75), respectively.
The results are given as means±standard errors. Statistical analysis was performed with the Mann-Whitney U-test for the nonparametric data to analyze differences between the two study groups. The relations between the continuous variables were evaluated with Spearman's rank correlation technique. Significance was determined at the 5% level. Multiple linear stepwise regression analysis was performed to assess the factors that may influence the serum leptin concentration. Statistical analysis was done with the statistical package for social science (SPSS).
The clinical and demographic characteristics of the COPD patients and healthy controls are shown in Table 1. The COPD patients had a significantly lower BMI, percent of body FM and percent of FFM than did the control subjects, whereas no difference was seen for the resting energy metabolism, which was expressed as a percentage of the predicted value (%REE). The patients with COPD had moderate to severe airflow limitation (GOLD I or II), but the healthy subjects had normal pulmonary function.
We analyzed the plasma leptin level and the activity of the TNF-α system, including the sTNF-R55 and sTNF-R75, in the COPD patients and the healthy subjects (Table 2). The plasma leptin levels were significantly lower in the patients with stable COPD than those in the healthy controls (1086±443.9 pg/mL vs. 1598.2±312.2 pg/mL, respectively, p<0.001). The plasma leptin levels that were corrected for the FM were not different between the patients with COPD and the healthy controls (78.3±30.9 pg/mL/kg vs 70.9±17.3 pg/mL/kg, respectively, p>0.05).
The plasma leptin concentration in the chronic bronchitis patients was significantly higher than that in the emphysema patients. However, there was no difference after adjustment for FM (Table 4). We found a significant correlation between the plasma leptin concentration and the BMI in the chronic bronchitis patients (r=0.866, p<0.001), but there was no such correlation in the emphysema patients.
Although the serum TNF-α levels were higher in the stable COPD patients than those in the controls, this difference was not statistically significant (8.5±3.1 pg/mL vs. 7.2±3.5 pg/mL, respectively, p>0.05). However, the sTNF-R55 and sTNF-R75 levels in the stable COPD patients were significantly higher than those in the healthy subjects (p<0.001).
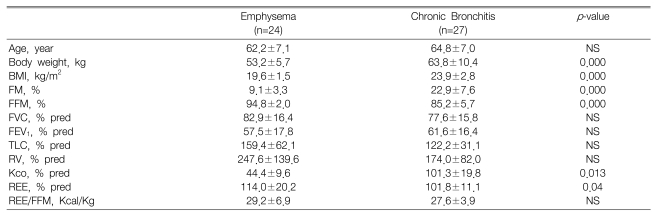
The characteristics of the COPD subtypes in the 27 patients with chronic bronchitis and the 24 patients with emphysema are presented in Table 3. For the patients with chronic bronchitis and emphysema, the FVC, FEV1, total lung capacity and residual volume were not different. Nonetheless, the difference of body composition between the two diseases was distinct. The BMI of the emphysema patients was significantly lower than that of the patients with chronic bronchitis (p<0.001). However, the FM, which was expressed as a percentage of the total body weight (%FM), was significantly higher for the patients with chronic bronchitis. This suggests that tissue depletion is more severe in emphysema patients.
The REE, which was expressed as a percentage of the predicted value, was higher for the patients with emphysema that for the patients with chronic bronchitis (p<0.05). However, there was no difference of the REE adjusted for the FFM (REE/FFM), which is the biologically active component, between the two subtypes of COPD.
The plasma TNF-α levels were higher in the chronic bronchitis patients than in the emphysema patients (9.1±3.4 pg/mL vs 8.4±3.2 pg/mL, respectively) but the difference was not statistically significant. The sTNF-R55 and sTNF-R75 levels were not different between the two subgroups of COPD (p>0.05) (Table 4).
First, we investigated the relationships between the plasma leptin concentration and body composition (Table 5). The leptin concentration was well correlated with the BMI and %FM of the patients with chronic bronchitis. We did not find significant correlations between the plasma leptin concentration and BMI in the emphysema patients. However, the plasma leptin level was correlated with the %FM (r=0.450, p=0.027).
Second, we analyzed the plasma leptin concentrations and the serum TNF-α and the sTNF-R55 and R-75 levels in the patients with COPD (Table 6). There was no correlation between the plasma leptin concentration that was adjusted for the fat mass and the serum sTNF-R55 and R-75 levels in the COPD patients, including the emphysema and chronic bronchitis patients. The TNF-α levels were not correlated with any components of the body composition in the COPD patients (data were not shown).
Until now, nutritional aspects such as the reduction of food intake, the impairment of amino acid metabolism and the increased energy metabolism have been considered to be important causes of tissue depletion in COPD patients1, 17-19). We assessed the body composition and the tissue depletion in patients with COPD. The purpose of this study was to determine the role of plasma leptin and the effect of the proinflammatory cytokine TNF-α system as a mechanism related to the tissue depletion and energy metabolism in clinically stable COPD patients.
In our study, the COPD patients showed significant tissue depletion compared to the healthy controls. Weight loss was severe in the emphysema patients. However, the pattern of tissue depletion was distinctly different according to the subtypes of COPD. The patients with emphysema have less body fat and this is composed of mostly FFM, whereas the body fat is relatively high in chronic bronchitis patients. Especially, there was no difference in BMI between chronic bronchitis patients and the healthy controls. Similar results have been reported by Engelen6) and Schols2, 20). These different wasting patterns suggest that diverse mechanisms may be involved in the pathogenesis of weight loss in COPD patients.
The mechanisms of weight loss in COPD patients are still unclear. Both anorexia and excessive energy expenditure play a role in the weight-losing associated with chronic wasting diseases1). Leptin is a pleiotrophic hormone that plays an important role in the regulation of food intake and energy homeostasis9, 10). In previous studies, the plasma leptin levels were significantly lower in the patients with COPD, and especially in those patients who were losing weight or who had a mal-nourished status, than the plasma leptin levels in the healthy controls20, 21). In another study, there was no difference in the concentrations of plasma leptin between the COPD patients without weight loss and who had clinically stable disease and those COPD patients with acute exacerbation22, 23). In this study, the plasma leptin concentrations that were corrected for fat mass were not different between the chronic stable COPD patients and the healthy controls, and plasma leptin concentrations were correlated with nutritional aspects. Because of their significant differences in fat-mass, the difference in the plasma leptin concentrations between the emphysema and chronic bronchitis patients could be easily explained. The positive correlation between plasma leptin and the BMI or FM in chronic stable COPD patients demonstrates that the plasma leptin concentrations remain controlled in stable COPD patients.
Some investigators postulate that hypermetabolism plays a role in the wasting associated with COPD. In COPD, the resting energy expenditure and/or daily energy expenditure may be elevated24, 25). The suggested mechanisms for hypermetabolism are the increased oxygen cost of breathing and the decreased mechanical efficacy during exercise26). Schols and colleagues20) searched for the resting energy expenditure in COPD patients and they found that the resting energy expenditure was not different in the subtypes of COPD patients. Our results are concordant with that previous study. In our study, the %REE was higher in the patients with emphysema than that in the chronic bronchitis patients. However, if corrected for the FFM which is the biological active determinant of the REE, no difference was noted between the two subtypes of COPD. These findings are contrary to other studies21, 22). In clinically stable patients with COPD, we were unable to find a relationship between hypermetabloism and weight loss.
It has been reported that the activity of the TNF-α system was significantly higher in the bronchial biopsies27) and induced sputum28) of COPD patients. In addition, increased TNF-α levels were demonstrated by Takabatake21, 29) and Di Francia30) in weight-losing, stable COPD patients. TNF-α is considered as a marker of systemic inflammatory reaction. There have been contradictory results of the TNF-α system in the pathogenesis of tissue depletion in COPD patients. Elevated plasma TNF-α levels were reported by some investigators in clinically stable COPD patients21) but not by other researchers, or these elevated plasma TNF-α levels were found only in the weight-losing30) or hypoxemic patients33). In our study, the activity of the TNF-α system, including TNF-R55 or TNF-R75, was found to be significantly elevated in the clinically stable COPD patients, as compared to healthy controls. However, there was no difference in the activity of the TNF-α system between the emphysema and chronic bronchitis patients. These results suggest that the activity of the TNF-α system may not involved with weight-lost in patients with stable COPD.
Experimental animal14, 15) and human34) studies have provided evidence for a link between leptin and proinflammatory cytokines. TNF-α and IL-1 treatment of fasted hamsters increased the concentration of leptin in circulation and the leptin messenger RNA in adipose tissue14). Zumbach and coworkers34) reported that administration of endotoxin or cytokines such as TNF-α or IL-1 produced a prompt and dose-dependent increase in the serum leptin levels in humans. In this study, although the plasma leptin levels were significantly higher in chronic bronchitis patients, the levels of plasma leptin levels corrected for fat-mass were not different between the emphysema and chronic bronchitis patients. Furthermore, we could not find any relationship between the plasma leptin concentration and the activity of the TNF-α system. However, there was positive correlation between the plasma leptin concentration and the BMI and FM. This correlation was stronger in patients with chronic bronchitis. These findings suggest that the plasma leptin concentration was primarily affected by the body composition, the BMI or the FM, rather than by the activity of the TNF-α system. In clinically stable COPD patients, the physiologic regulation mechanism of leptin was maintained normally in the condition with weight loss. In previous studies, any relationship between the plasma leptin concentration and the activation of the TNF-α system as related to systemic inflammation was contradictory. Some authors, but not others, have reported the positive correlation between the plasma leptin level and the TNF-α system in clinically stable COPD patients.
In summary, we found that the plasma leptin levels were not elevated in clinically stable COPD patients and the levels were correlated with body composition parameters such as the body mass index and the fat-mass in weight losing, stable COPD patients. There was no difference in the activity of the TNF-α system between the emphysema patient and relatively weight losing, chronic bronchitis patients. Moreover, there was no correlation between the plasma leptin levels and the TNF-α system.
In conclusion, the normal regulation mechanism of leptin is maintained in chronic stable COPD patients. The interaction of leptin and the activity of the TNF-α system in the pathogenesis of tissue depletion may not play an important role in chronic stable COPD patients.
References
1. Schols AM, Soeters PB, Mostert R, Saris WH, Wouters EF. Energy balance in chronic obstructive pulmonary disease. Am Rev Respir Dis 1991. 143:1248–1252PMID : 2048807.


2. Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 1993. 147:1151–1156PMID : 8484624.


3. Rochester DF, Braun NM. Determinantsof maximal inspiratory pressure in chronic obstructive pulmonary disease. Am Rev Respir Dis 1985. 132:42–47PMID : 4014871.

4. Wilson DO, Roger RM, Writer EC, Anthonisen NR. Body weight in chronic obstructive pulmonary disease. Am Rev Respir Dis 1989. 139:1435–1438PMID : 2658702.


5. Schols AM, Mostert R, Soeter PB, Wouters EF. Body compositionexercise performance in patients with chronic obstructive pulmonary disease. Thorax 1991. 46:695–699PMID : 1750015.



6. Engelen MP, Schols AM, Lamers RJ, Wouters EF. Different patterns of chronic tissue wasting among patients with chronic pulmonary disease. Clin Nutr 1999. 18:275–280PMID : 10601534.


7. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects ofprotein encoded by the obese gene. Science 1995. 269:543–546PMID : 7624777.


8. Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper KJ, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996. 84:491–495PMID : 8608603.


9. Flier JS. Leptin expression and action: new experimental paradigms. Proc Natl Acad Sci U S A 1997. 94:4242–4245PMID : 9113973.



11. Fraggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J 2001. 15:2565–2571PMID : 11726531.


12. Merabet E, Dagogo-Jack S, Coyne DW, Klein S, Santiago JV, Hmiel SP, Landt M. Increased plasma leptin concentration in end-stage renal disease. J Clin Endocrinol Metab 1997. 82:847–850PMID : 9062494.


13. Carlson GL, Saeed M, Little RA, Irving MH. Serum leptin concentrations and their relation to metabolic abnormalities in human sepsis. Am J Physiol 1999. 76:E658–E662PMID : 10198301.

14. Grunfeld C, Zhao C, Fuller J, Pollock A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamster. J Clin Invest 1996. 97:2152–2157PMID : 8621806.



15. Sarraf P, Frederich RC, Turner EM, Jaskowiak NT, Rivet DJ 3rd, Flier S, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin level: potential role in inflammatory anorexia. J Exp Med 1997. 185:171–175PMID : 8996253.



16. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995. 152:s77–s121PMID : 7582322.

17. Donahoe M, Rogers RM, Wilson DO, Pennock BE. Oxygen consumption of the respiratory muscles in normal and in malnourished patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1989. 140:385–391PMID : 2764376.


18. Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998. 157:1791–1797PMID : 9620907.


19. Yoneda T, Yoshikawa M, Fu A, Tsukaguchi K, Okamoto Y, Takenada H. Plasma levels of amino acids and hypermetabolism in patients with chronic obstructive pulmonary disease. Nutrition 2001. 17:95–99PMID : 11240335.


20. Schols AM, Creutzberg EC, Buurman WA, Campfield LA, Saris WH, Wouters EF. Plasma leptin is related proinflammatory status and dietary intake in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999. 160:1220–1226PMID : 10508810.


21. Takabatake N, Nakamura H, Abe S, Hino T, Saito H, Yuki H, Kato S, Tomoike H. Circulating leptin in patients with chronic bstructive pulmonary disease. Am J Respir Crit Care Med 1999. 159:1215–1219PMID : 10194168.


22. Karaka S, Karadag F, Karul AB, Gurgey O, Gurel S, Guney E, Cildag O. Circulating leptin and body composition in chronic obstructive pulmonary disease. Int J Clin Pract 2005. 59:1167–1170PMID : 16178984.


23. Yang YM, Sun TY, Liu XM. The role of serum leptin and tumor necrosis factor-α in malnutrition of male chronic obstructive pulmonary disease patients. Chin Med J 2006. 119:628–633PMID : 16635406.


24. Creutzberg EC, Wouters EF, Vanderhoven-Augustin IM, Dentener MA, Schols AM. Disturbances in leptin metabolism are related to energy imbalance during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000. 162:1239–1245PMID : 11029324.


25. Baarends EM, Schols AM, Pannemans DL, Westerterp KR, Wouters EF. Total free living energy expenditure in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997. 155:549–554PMID : 9032193.


26. Schols AM. TNF-α and hypermetabolism in patients with chronic obstructive pulmonary disease. Clin Nutr 1999. 18:255–257PMID : 10601531.


27. Mueller R, Chanez P, Champll AM, Bonsquet J, Heusser C, Bullock GR. Different cytokine patterns in bronchial biopsies in asthma and chronic bronchitis. Respir Med 1996. 90:79–85PMID : 8730325.


28. Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 1996. 153:530–534PMID : 8564092.


29. Takabatake N, Nakamura H, Abe S, Inoue S, Hino T, Saito H, Yuki H, Kato S, Tomoike H. The relationship between chronic hypoxemiaactivation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000. 161:1179–1184PMID : 10764309.


30. Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994. 150:1453–1455PMID : 7952575.


31. Schols AM, Buurman WA, Staal van den Brekel AJ, Dentener MA, Wouters EF. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 1996. 51:819–824PMID : 8795671.



32. de Godoy I, Donahae M, Calhoun WJ, Mancino J, Roger RM. Elevated TNF-alpha production by peripheral blood monocytes of weight-losing COPD patients. Am J Respir Crit Care Med 1996. 153:633–637PMID : 8564110.


Table 1
Characteristics of the study patients with chronic obstructive pulmonary disease and the healthy subjects

Table 2
Concentration of serum leptin, tumor necrosis factor-α, plasma sTNF-R55 and sTNF-R75 in patients with chronic obstructive pulmonary disease and the healthy subjects

Table 4
Concentration of serum leptin, tumor necrosis factor-α, plasma sTNF-R55 and sTNF-R75 in patients with emphysema or chronic bronchitis






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



