INTRODUCTION
The toxic effects of acute mercury vapor inhalation have been described1). The clinical picture evolving may be divided into three phases. The initial phase is manifested as a flu-like illness. The intermediate phase involves a period in which severe multi-organ symptoms may manifest. The late phase consists of a period when central nervous symptoms persist. Rare cases with very high acute exposure to mercury vapor have been reported, in which severe respiratory symptoms dominate the clinical picture2). The cause of death in all lethal cases is progressive respiratory failure and the pathologic findings in the lungs at autopsy reveal various stages of acute lung injury, similar to those found in the acute respiratory distress syndrome (ARDS)3).
We report the findings in a patient with severe mercury inhalation injury who manifested all the three phases of the clinical picture, including ARDS, and yet survived with treatment using corticosteroid and penicillamine.
CASE REPORT
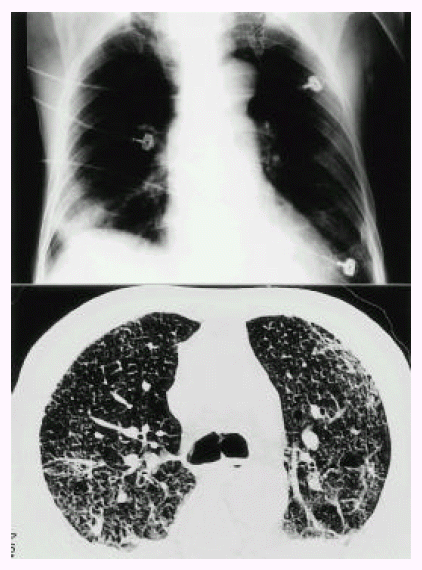
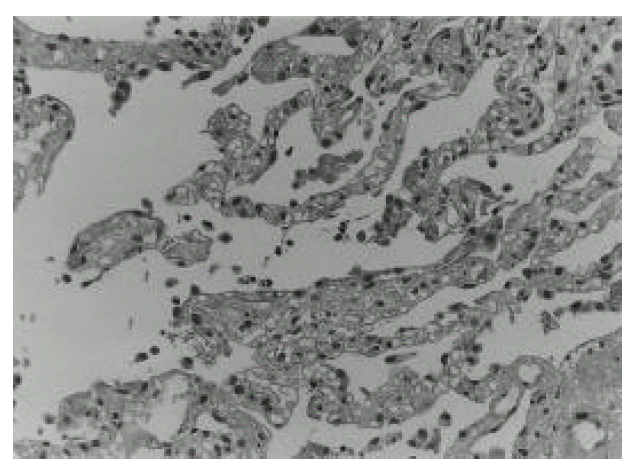
On admission day, a 72-year-old man had attempted illicit use of mercury-lead amalgam to treat hemorrhoids. This procedure was done in a closed room of his house. He became ill with paroxysmal cough, dyspnea, chest pain, nausea and vomiting, but he was unaware of the cause of his illness. He was admitted and then progressively developed dyspnea and respiratory failure. Routine blood chemistries were unremarkable with the exception of arterial blood gases at room air, which revealed pH 7.533, PaO2 25 mmHg, and PaCO2 21 mmHg. His initial chest roentgenogram was normal. On the second day after exposure, chest radiography revealed bilateral diffuse pulmonary infiltrates (Fig. 1. Top) and bilateral air-space consolidation on high resolution computerized tomography of chest (HRCT), especially in the dependent portion (Fig. 1. Bottom). Transbronchial lung biopsy revealed a chemical pneumonitis, suggestive of pulmonary change of early acute lung injury induced by the exposure to mercury vapors (Fig. 2). The initial urinary mercury concentration was 6402 ug/L. On the third day after exposure to mercury vapor, therapy was initiated with mechanical ventilation and intravenous methylprednisolone pulse (500 mg/day) for 3 days, followed by oral prednisolone (1 mg/kg) and oral D-penicillamine (1500 mg/day) for 7 days. The follow-up chest X-ray showed a much-improved state (Fig. 3. Top) and follow-up urinary mercury concentration was 25.6 ug/L. Follow-up arterial blood gases at room air revealed PaO2 64.9 mmHg, PaCO2 31 mmHg, pH 7.48. He improved daily. But on the 16th day after exposure to mercury vapor, he became disoriented. On physical examination, his mental status was disoriented and auscultation revealed fine crackle bilaterally. Brain MRI showed old cerebral infarction in the right frontal lobe, and a follow-up HRCT in chest showed interstitial fibrosis of both lung fields, especially in the dependent portions (Fig. 3. Bottom). He was treated with second intravenous methylprednisone pulse (500 mg/day) for 3 days and then oral prednisolone (1 mg/kg). Dyspnea improved slightly but his mental status was aggravated. Pulmonary symptoms showed a steady state, but mental status did not improve. He was discharged on the 21st day against medical advice.
DISCUSSION
Elemental mercury vapor is absorbed rapidly through the alveolar membrane and transported by blood to the brain and other parts of the nervous system1). Mercury is rapidly converted to mercuric ions (Hg++), which are then excreted in the urine and feces. Elimination of elemental mercury occurs primarily in the urine with a half-life of about 60 days. After oxidation, elemental mercury may act as mercuric ion or divalent mercury and thus may be identical to the chemical form that occurs after dissociation of mercuric salts. The danger of mercury vapor is similar to that of ingestion of mercuric chloride or the mercurial diuretics. The same areas of the brain are affected by both inorganic and organic mercury. The tremor, rigidity, truncal unsteadiness and impaired gait may produce a Parkinsonian syndrome suggesting involvement of the basal ganglia and the cerebellum. Involvement of the corpus callosum may be indicated by performance on tests showing no improvement on switching to the preferred from the nonpreferred hand. Defects in memory suggest involvement of the temporal lobe. A urine excretion level of 300mg/L probably represents mercury poisoning; 100 mg/L of mercury in urine requires treatment and levels of 50 mg/L or lower are considered safe. However, urinalysis often yields unreliable results and normal levels have not been clearly established as yet. In our case, the urinary mercury concentration was 6408 ug/L, a level representing acute mercury intoxication.
A clinical picture evolves that may be divided into the following three phases:1) The initial phase (first few days after exposure) is manifested as metal fume fever or a flu-like illness characterized by chills, fever, aching muscles, dryness in the mouth and throat and headache. Toxic pneumonitis with respiratory failure complicate the picture to revese cases, such as the case presented. The intermediate phase (symptoms present 2 weeks after an accident) can be defined as the period during which severe multiorgan symptoms (central nervous system, respiratory tract, gastrointestinal and renal systems) may be manifested. In the respiratory tract, mercury vapour acts as a direct airway irritant and a cellular poison. In mercury vapour inhalation, death has occurred from respiratory failure. Postmortem studies have shown severe damage to the bronchi and bronchioles with marked alveolar edema2). In the presence of necrosis, complications such as interstitial emphysema, pneumomediastinum and pneumothorax can occur4). The late phase involves the period when central nervous symptoms persist and other organ symptoms resolve. Thus, acute exposure to elemental mercury and its vapor induce acute inorganic mercury toxicity and can cause long-term, probably irreversible, neurologic sequelae. The clinical course of our case showed all three phases.
The pathologic studies show that the histologic picture varied in accordance with how long each patient survived postinsult. Kanluen et al3). reported that the pathologic findings in the lungs at autopsy reveal various stages of acute lung injury. In our case, at 5 days postinsult, transbronchial lung biopsy showed early acute lung injury, characterized by slight pneumocyte hyperplasia and intra-alveolar fibrin clot with hyaline membrane formation. The consolidation areas on radiograph were predominantly arranged in edematous interstitium. There was also the deposition of gray-black granular pigments in the alveolar septae, highly suggestive of mercury pigments. A minimal interstitial neutrophilic and mononuclear infiltrate was also present. At 16 days postinsult, HRCT showed moderated air space consolidation and markedly increased interstitial fibrosis, especially in previously edematous portions. Also, emphysema was present in the upper lung field. Lung biopsy was not performed due to the patientŌĆÖs altered mental status. These histologic changes are similar to changes described in the adult respiratory distress syndrome from other causes2).
The therapeutic aspect of our case also deserves mention. Corticosteroids have been used sporadically, as reported in the literature2). The response to corticosteroids may have been coincidental given the absence of response in the adult respiratory distress syndrome in previous studies. In contrast, we saw beneficial positive response in our case. It has been suggested that steroids may prevent progression to severe interstitial fibrosis if used in mildly affected patients. But the progression of late stage occurred rapidly as we tapered the steroid.
The effectiveness of chelating agent for mercury-induced pulmonary toxicity remains unclear. Penicillamine is generally accepted as an effective chelating agent for mercury5). Dimercaptopropanol (BAL) is also effective; however, penicillamine has the advantage of oral administration and is possibly more potent. D-penicillamine, as an oral compound, may be useful in the less severe symptomatic inorganic and elemental mercury inhalation exposures, but its actual value remains to be determined by clinical studies. D-penicillamine reverses sulfhydryl binding in the blood and chelates both mercury and lead. N-acetyl-d,L-penicillamine has been administered successfully to patients with inorganic mercury-induced neuropathies (tremor, ataxia) and chronic elemental mercury toxicity1). Although chelation therapy has been shown to decrease serum mercury concentration, review of the literature shows that this has no effect on progression of acute lung injury. Jaeger et al6) postulated that lung tissue damage is complete and that the treatment of serum levels with chelating agents has no effect on the reversal of lung damage. In Rowens et al2) reports, despite reduction in serum mercury levels with dimercaprol, there was also no reversal in the progression of lung injury and respiratory dysfunction. Although a number of authors have advocated the use of N-acetyl-d,L-penicillamine, this modified chelating agent is not currently available in North America7). Even though it has been reported successful chelation, the effectiveness of D-penicillamine therapy remains unclear.
In summary, we report a patient with acute respiratory distress syndrome after illicit use of mercury vapor for hemorrhoid treatment, who developed acute chemical pneumonitis.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





