INTRODUCTION
Pylephlebitis is defined as septic thrombophlebitis of the portal vein or one of its tributaries, usually secondary to suppuration either in the region drained by the portal venous system or in structures contiguous to the portal vein (e.g., the common bile duct)1). Pylephlebitis, that previously well-characterized complication of appendicitis, is rare now, owing to core expeditious surgical management of intra-abdominal infections and the advent of antibiotic therapy2). Despite this rarity, mortality remains high and the number of case reports dealing with this entity seems to have increased over the past 15 years3). The current widespread use of abdominal ultrasonography and CT may, in part, explain the recent increase in reported cases. We report on a case of pylephlebitis and multiple liver abscesses secondary to appendicitis.
CASE REPORT
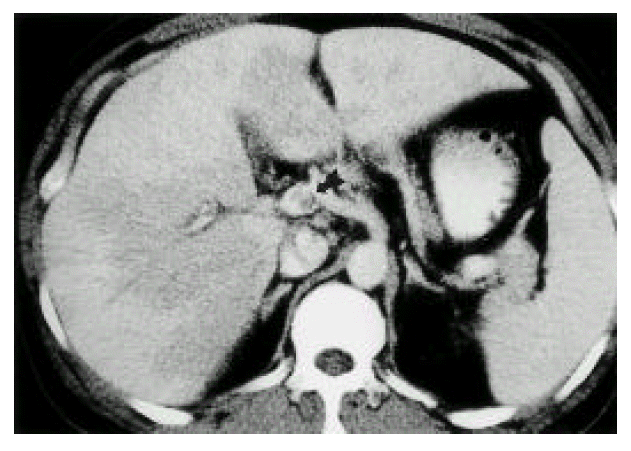
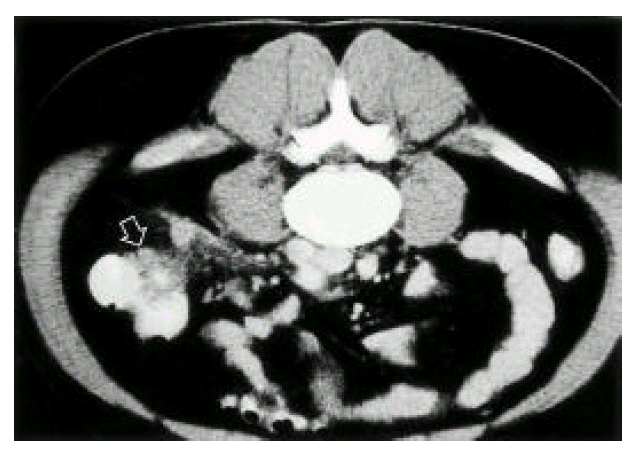
The 37-year-old man had a 5-days duration of fever, chills, small amount of watery diarrhea and yellowish skin discoloration. His past medical and surgical history was unremarkable, but he recalled an episode of diffuse abdominal pain, nausea and vomiting more than 3 weeks earlier. He developed fever (38.8°C) without any focal symptoms. Bowel sounds were slightly increased and minimal right upper and lower quadrant tenderness was presented. Peripheral white blood cell count was 11,300/mm3 (segment neutrophil: 66%, metamyelocyte: 20%). Hematocrit was 27.4% and platelet count was 76,000/mm3. Other values were SGOT 68 IU/L, SGPT 52 IU/L, gamma-GPT 68 IU/L, alkaline phosphatase 152 IU/L, total-bilirubin 16.79 mg/dL, direct—bilirubin 14.30 mg/dL, protein 5.54 g/dL and albumin 2.81 g/dL. Abdominal ultrasonography revealed a thrombus in the upper SMV, lower extrahepatic portal vein and dilated SMV. Hepatic vein and IVC were not remarkable. Doppler studies showed antegrade flow in the portal vein and space-occupying lesions in the liver. GB was collapsed with diffuse wall thickening. The pancreas and kidneys appeared normal. Bactroides fragilis was grown from blood culture. No micro-organism was cultured from stool and urine. Since intra-abdominal septic foci were suspected, abdominopelvic CT scan was taken. A contrast-enhanced CT scan confirmed the portal vein thrombosis and also demonstrated superior mesenteric vein thrombosis(Fig. 1). Abnormal multiple focal densities located in the liver were multiple, small septic emboli. Dirty fat sign, with streaky infiltration and enlarged lymph nodes, were noted in the right lower abdomen, adjacent to the cecum. Normal appendix was not visible. An inflammatory condition around the cecum, such as chronic perforated appendicitis, was highly suggested(Fig. 2). A small amount of abnormal fluid was seen around the liver, right paracolic gutter and in the pelvis(Fig. 2). On the basis of the microbiological data, treatment was done with imipenem alone 0.5g/Iv q 6hours. On the 7th hospital day, the patient’s condition continued to improve and he was discharged 4 weeks later. Follow-up CT scan showed minor residual pericolonic inflammation, resolution of the multiple liver abscess and improvement of the portal vein thrombosis. After 14 days from treatment with imipenem, his bilirubin level was lowered to near-normal level (total-bilirubin 2.5 mg/dL, direct-bilirubin 1.3 mg/dL). He recovered. Further treatment was not needed.
DISCUSSION
Pylephlebitis or Septic thrombophlebitis of the portal vein, a precursor of liver abscess, is an extremely rare and frequently fatal complication of diverticulitis.
In 1846, Waller documented autopsy findings in a patient with appendicitis, including multiple liver abscesses and pylephlebitis. He concluded that appendicitis was the direct cause of pylephlebitis and liver abscess. By the turn of the century, the incidence of pylephlebitis following appendicitis gradually diminished with the advent of antibiotics and early surgical treatment. Subsequently, acute colonic diverticulitis has become the leading cause of suppurative pylephlebitis4). The true incidence of pylephlebitis is difficult to estimate, however, since the diagnosis may be obscured by either the inciting focus of infection or the liver abscesses resulting from “portal pyemia”, such as acute diverticulitis, intestinal perforation, uterine infection, suppurative pancreatitis and pancreatic abscess formation, splenic abscess, perirectal abscess, hemorrhoid, epididymitis, omphalitis and inflammatory bowel disease2). In our case, appendicitis was the cause of pylephlebitis. Furthermore, the high morbidity and mortality of suppurative pylephlebitis and intrahepatic abscesses are, at least in part, attributable to the nonspecificity of the symptoms and signs and the limitations of available diagnostic modalities5). A clinician must be cognizant of both the nonspecificity of the clinical presentation and the limitations of the sensitivity of these diagnostic modalities. Nothing has superseded the necessity for a high index of clinical suspicion regarding these diagnoses. Diagnoses are based on clinical features, laboratory findings, blood culture and radiological examinations, or postmortem examination. Clinical feature includes fever, right upper quadrant pain, diarrhea, jaundice and hepatomegaly2). The clinical “pearl” that jaundice is rare in pylephlebitis, except in cases complicated by multiple liver abscesses, appears to be valid. Jaundice developed late (no earlier than 5 days after the onset of symptoms) since the relative absences of jaundice early in the illness helps distinguish pylephlebitis from ascending Escherichia coli, followed by Proteus mirabilis, Klesiella pneumoniae, Enterobacer species, Pseudomonas species and gram-positive cocci (Staphylococcus aureus, Streptococcus species). More recently, coloenteric anaerobic bacteria (Clostridia, Bacteroides, Fusobacterium and anaerobic streptococcus species) have been isolated with increasing frequency, as a consequence of both improved culture techniques and selective pressure by the prevalent use of antibiotics5). In our case, Bacteroides fragilis was grown from blood. Modern radiological imaging techniques provide supportive diagnostic evidence7). With regard to diagnostic imaging in pylephlebitis, the current series suggests that ultrasonography is a useful modality for demonstrating portal vein thrombosis. CT scanning also shows promise in this regard, and may be less operator-dependent than ultrasonography. In the setting of probable intra-abdominal infection, CT scanning may be the most reasonable initial choice for imaging, given its proven ability to detect not only thrombi but pericolonic abscesses as well8). Serial CT scans document both the outcome and temporal course of secondary hepatic infection. Early in the disease process, hepatic infection and inflammation are microscopic, and contrast CT may demonstrate patchy areas of attenuated hepatic parenchyma. Later in the clinical course, parenchymal hepatic infection may coalesce to form multiple microabscess, or a single macroabscess amenable to percutaneous CT-guided drainage9). Also in our case, inhomogenous patchy infiltration in the hepatic parenchyme was observed at the time of diagnosis, and resolution of these lesions were observed in follow-up film. Although modern imaging techniques have allowed the early diagnosis of pylephlebitis, the patient’s history and physical examination still provide clues to the diagnosis. On the basis of treatments, it appears that empirical antibiotic therapy for a patient with suspected pylephlebitis should include broad coverage for enteric facultative gram-negative bacilli and agents active against anaerobes, especially B. fragilis, and coverage for aerobic Streptococcus species. Specific coverage for enterococci does not appear to be a necessity. The ideal duration of antibiotic therapy for pylephlebitis is unclear2). Given the frequency of liver abscess as a complication of pylephlebitis, however, a minimum 4 weeks of therapy seems prudent since developing abscesses may not be visualized on CT scans. Patients with demonstrated macroscopic liver abscess complicating pylephlebitis should probably receive at least 6 weeks of antibiotic therapy, with or without drainage. In our case, imipenem (2.0g/d) monotherapy was performed for 2 weeks and resulted in complete resolution of hepatic lesions. No further treatment was performed. The role of anticoagulation in the treatment of pylephlebitis is controversial10). In prior writings, descriptions of its role have ranged from “unnecessary” to “probably useful” to “recommended”. All of these opinions appear to have been based on limited data and personal experience, since no formal study of anticoagulation in pylephlebitis has ever been done. Despite such evidence that heparin may not be critical for the survival of patients with pylephlebitis, the possibility exists that anticoagulation might benefit some patients by decreasing the chance of septic embolization to the liver from infected portal thrombi and pulmonary emboli2). Surgical intervention for pylephlebitis typically involves opening and drainage of the focus of infection, separation of large vessels of the portal system from the focus of infection (i.e., pericolonic or hepatic abscess) rather than surgery on the infected vessels themselves11). In our patient, a previously healthy patient presented with nonspecific symptoms and fever. Bacteroides fragilis was grown from blood, which clearly suggested an intra-abdominal origin. Appendicitis was the most likely cause of sepsis, confirmed by abdominal CT, with seeding to the portal vein and liver. Septic conditions were improved with early initiation of broad-spectrum antibiotics. No surgical intervention was performed, since the intra-abdominal phlegmonous process responded to antibiotic therapy.
Outcome depends on multiple factors, including age greater than 70 years1), multiple liver abscess2), combination of hepatic abscess and pylephlebitis3), concomitant intrahepatic and extrahepatic abscesses, usually subphrenic4), deterioration of liver function5) due to intrahepatic infection, particularly jaundice, and hypoalbuminemia1).
Although radiological imaging studies (e.g. contrast-enhanced abdominopelvic CT scan) facilitates diagnoses, early suspicion and prompt antibiotic therapy can lead to resolution of portal vein thrombosis and multiple liver abscesses thus resulting in recovery.
In conclusion, pylephlebitis is an uncommon complication of suppurative infections (most often diverticulitis) in the portal drainage area. Although rare, pylephlebitis is a treatable but often lethal complication of intra-abdominal sepsis, pylephlebitis should be suspected in patients with intra-abdominal sepsis with liver function abnormalities. A high index of suspicion is required for making an expedient diagnosis. The timely administration of broad-spectrum antibiotics appears to be the most critical component of therapy for pylephlebitis.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





