 |
 |
| Korean J Intern Med > Volume 14(2); 1999 > Article |
|
Abstract
Objectives
The aim of this study was to evaluate changes of body composition in cirrhotic patients. Dual energy x-ray absorptiometry (DEXA) and anthropometry were used, and the values obtained were compared.
Methods
Mid-arm fat and muscle areas were calculated by anthropometry in 66 cirrhotic patients and 94 healthy controls. In 37 of the cirrhotic patients and 39 of the controls, fat mass, lean soft tissue mass and bone mineral contents were measured with DEXA.
Results
The number of cirrhotic patients with measured values below the fifth percentile of normal controls was 21 (31.8%) by mid-arm fat area, six (9.1%) by mid-arm muscle area, 15 (40.5%) by fat mass and 0 (0%) by lean soft tissue mass. The fat mass in cirrhotic patients was less than in controls, whereas lean soft tissue mass and bone mineral content were not different. Fat depletion was severe in Child-class C patients and with severe ascites. Mid-arm fat area and fat mass showed close correlation (r = 0.85, p<0.01), but mid-arm muscle area and lean soft tissue mass showed poor correlation (r = 0.32, p<0.05).
More than 60% of patients with liver cirrhosis suffer from malnutrition1), and this may adversely affect morbidity and mortality in liver cirrhosis2–4). Proper nutrition may reduce complications and increase survival rates5–7), though accurate, quantitative nutritional assessment is difficult in patients with liver cirrhosis; conventional markers, such as weight, serum albumin levels and peripheral lymphocyte counts, are influenced by non-nutritional factors8).
Changes in one or more body components not only provide early detection of malnutrition but also show the pattern of specific tissue loss, and this might provide insight into the disease process and information regarding outcome and prognosis9,10). The methods used include anthropometric assessment, bioelectric impedance analysis, isotope dilution, the measurement of total body potassium, dual energy x-ray absorptiometry (DEXA) and neutron activation11). Neutron activation analysis, DEXA or deuterium oxide dilution accurately reflect changes in body composition associated with chronic liver disease9). Because of availability problems, cost and the amount of radiation involved, the clinical use of neutron activation analysis is limited12). DEXA measures three body components: fat mass, lean soft tissue mass (comprising muscle, inner organs and body water) and bone mineral content, and is a quick, simple method for body composition analysis. Its advantages are the relatively low radiation dose, excellent reproducibility and high precision11,12). In clinical practice, the most reliable method of nutritional assessment is anthropometric measurement. It is simple, and may be used as a bedside tool13), but its drawback is the potential of underestimation of fat loss in cirrhotic patients with subcutaneous fluid retention9). Measurements need to be validated by comparison with the results obtained by reference methods.
In this study, anthropometric measurements and DEXA were chosen because the former are quick, and easily performed at the bedside, and the latter accurately reflects changes in body composition associated with chronic liver disease11). We analysed the changes of body composition of patients with liver cirrhosis and compared them with those for healthy control subjects. Body composition was determined using anthropometry for fat and fat-free mass and DEXA for body fat, lean soft tissue mass and bone mineral content. The anthropometric findings for lean and fat tissue mass in cirrhotic patients were compared with those for total fat mass and lean soft tissue mass measured by DEXA.
Sixty-six patients (58 males, 8 females) with liver cirrhosis and 94 healthy controls (49 males, 45 females) were enrolled in this study. Their mean age was 51.8 (range 30–67) years and 52.9 (range 40–68) years, respectively. Control group consisted of volunteers who were considered healthy on the bases of history taking, physical examination and blood chemistries. The liver cirrhosis was diagnosed with liver function tests, abdominal CT or ultrasonography and clinical evidence of portal hypertension such as varices, splenomegaly or ascites. Patients with recent gastrointestinal hemorrhage, infection or malignancy were excluded. The severity of ascites was graded as mild or non-existent (detectable only by ultrasonography), moderate (obvious but less than tense) or severe (tense). In the cirrhotic group, ascites was mild or non-existent in 20 patients, moderate in 35 and severe in 11. In those with ascites or edema, all evaluation was made after disappearance of ascites and edema with medical treatment. Liver cirrhosis was virus-related in 44 cases and alcohol-related in 22. Child-Pugh scores showed that 25 were Child-class A, 24 were class B and 17 were class C. Anthropometry was performed in all cases, but DEXA was measured only in 37 cirrhotic patients and 39 controls who agreed with that test. The retrograde analyses of clinical parameters, such as age, body weight, body mass index and Child-classes detected no difference between the DEXA group and the non-DEXA group in both patient and control groups (Table 1).
Anthropometric measurements of mid-arm circumference (MAC) and triceps skinfold thickness (TSF) were obtained using a skinfold caliper (Fabrication Enterprises Incorporated, New York, USA) and a flexible steel tape. Measurements were taken midway between the tip of the acromion and olecranon process of the left arm, while the patient stood in a relaxed position17). Upper mid-arm muscle area (MAMA) and upper mid-arm fat area (MAFA) were calculated as14),
Using a total-body DEXA scanner (XR-26, MARK II), fat mass, lean soft tissue mass and bone mineral content were measured, and DEXA data and those of MAMA and MAFA measured by anthropometry, were compared between cirrhotic patients and healthy controls. Correlations between fat mass vs. MAFA and lean soft tissue mass vs. MAMA in patients and controls, which had been performed by anthropometry and DEXA, were evaluated using Pearson’s correlation coefficient.
To evaluate the clinical parameters related to depleted body components in cirrhotic patients, the numbers of patients, whose MAFA values were lower than the fifth percentile of controls, were counted and correlations with Child-classification and the severity of ascites were analysed. A p value of less than 0.05 was considered significant.
In cirrhotic patients of both sexes, fat mass and MAFA were lower than in sex-matched controls (p<0.05, Tables 2, 3, Figs. 1, 2). The number of cirrhotic patients with measured values below the fifth percentile of normal controls was 21 (31.8%) on the basis of MAFA and 15 (40.5%) on the basis of fat mass Fat depletion was more severe in Child-class C patients and those with severe ascites (Figs. 3, 4).
In cirrhotic patients and normal controls, lean soft tissue mass and MAMA were comparable (p>0.05, Tables 2, 3, Figs. 5, 6). The number of patients with cirrhosis with measured values below the fifth percentile of normal controls was six (9.1%) according to MAMA and 0 (0%) according to lean body mass.
Bone mineral content in cirrhotic patients and normal controls was comparable (Fig. 7).
This study, which investigated body compositional changes in patients with cirrhosis, demonstrated that there was a reduction in one or more body compositions in more than 40% of patients. It was very striking that the majority of patients showed predominant fat depletion despite maintaining their lean soft tissue mass and bone mineral content. Changes in body composition measured by anthropometry revealed as MAMA being impaired more severely than MAFA in males, whereas the opposite was true in females13,15). Crawford et al. reported that the majority of patients, who maintained body cell mass but had reduced body fat, were Child’s class A10). It is well known that body adipose deposits is the most important fuel source for patients with insufficient calorie intake16,17). A moderate nutritional disturbance affects only the fat component; in patients with liver cirrhosis, intestinal fat absorption may be abnormal18).
There are some methodologic problems in the evaluation of body cell mass in patients with liver cirrhosis. For example, the usefulness of DEXA for determining body cell mass in liver cirrhosis is controversial19,20). Because of extracellular water retention, lean soft tissue mass may not represent body cell mass, even if there was no evidence of overt ascites or fluid retention21). One study9) compared different methods for assessment of body composition in patients with liver cirrhosis, and found that in Child-class C patients, DEXA gave higher values for lean soft tissue mass than did neutron activation analysis. Anthropometry, furthermore, did not accurately reflect changes in fat-free mass. As shown by Bhatla et al.22) in patients on continuous ambulatory peritoneal dialysis, DEXA included excess body water in lean soft tissue mass and, where there is overhydration, may mask malnutrition. Some authors, however, have recommended DEXA for body composition analysis in stable dialysis patients23,24) or those with liver cirrhosis25) after ascites has been controlled. Prijatmoko et al9). showed that DEXA can detect advanced protein depletion in patients with liver cirrhosis. The methods used in this study, DEXA and anthropometry, tend to overestimate fat-free mass. Changes in the hydration status of soft tissues can alter attenuation of the dual energy source, resulting in incorrect calculation of the amount of lean tissue.
With regard to bone mineral content in cirrhosis, the findings differ. Prijatmoko et al9). showed that this was maintained, but other investigaors found that it was reduced26,27).
MAMA and MAFA measured by anthropometry represent muscle mass and fat and, to validate this system for fat mass measurement, we compared MAFA with fat mass. As observed previously9,28,29), MAFA values correlated closely with those of fat mass, but those of MAMA correlated poorly with lean soft tissue mass.
The nutritional status of cirrhotic patients with severe ascites and those who were Child-class C was more impaired, as reported by others5,13,14,30). Closer nutritional evaluation of patients with severe ascites and/or severe hepatic dysfuction is therefore required.
In summary, we have shown that, in more than 40% of patients with liver cirrhosis, there were reductions in the amount of stored fat, and that these changes were more severe in Child-class C patients and those with severe ascites. Fat mass estimated according to MAFA correlated closely with fat mass measured by DEXA. In view of its easy bedside use, MAFA is recommeded for the measurement of fat mass in cirrhotic patients.
Fig. 1.
Comparison of mid-arm fat area (MAFA) measured by anthropometry between 66 cirrhotic patients and 94 controls. Raw values and means were noted. LC, liver cirrhosis.
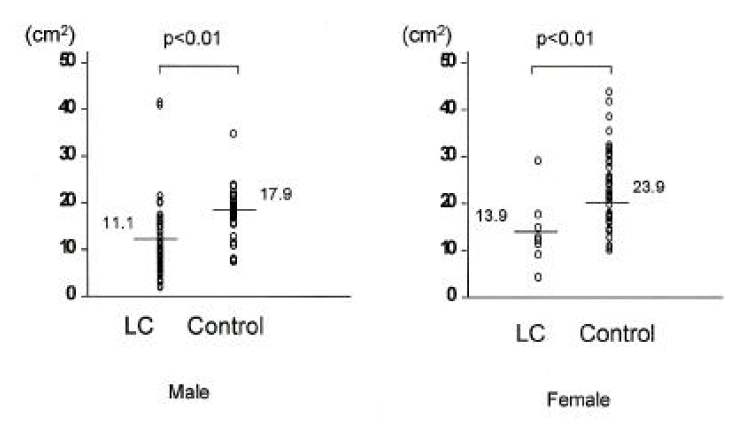
Fig. 2.
Comparison of fat mass measured by dual energy x-ray absorptiometry between 37 cirrhotic patients and 39 controls. Raw values and means were noted. LC, liver cirrhosis.
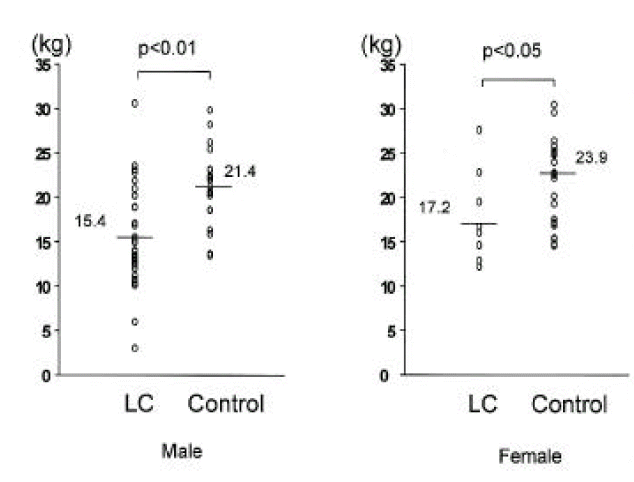
Fig. 3.
The mid-arm fat area (mean±SD) according to Child-class in 66 cirrhotic patients.
*, p< 0.05 vs. Child-class A.
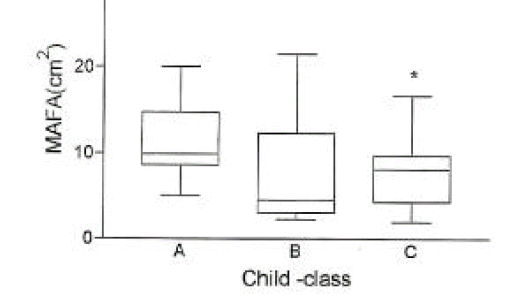
Fig. 4.
The mid-arm fat area (mean±SD) according to the degree of ascites in 66 cirrhotic patients. *, p< 0.05 vs. mild or none, and moderate ascites.
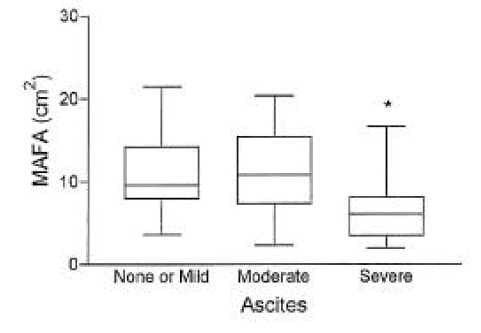
Fig. 5.
Comparison of mid-arm muscle area measured by anthropometry between 66 cirrhotic patients and 94 controls. Raw values and means were noted. LC, liver cirrhosis.
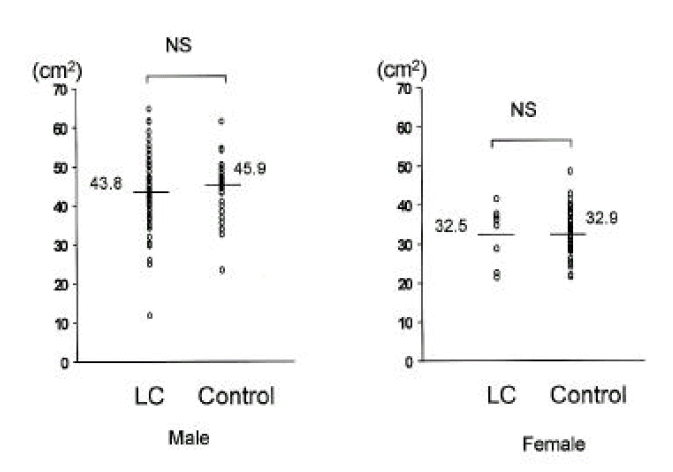
Fig. 6.
Comparison of lean body mass measured by dual energy x-ray absorptiometry between 37 cirrhotic patients and 39 controls. Raw values and means were noted. LC, liver cirrhosis.
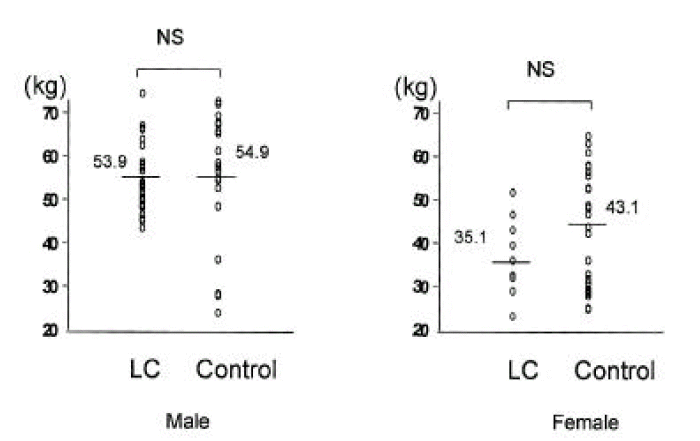
Fig. 7.
Comparison of bone mineral content measured by dual energy x-ray absorptiometry between 37 cirrhotic patients and 39 controls. Raw values and means were noted. LC, liver cirrhosis.
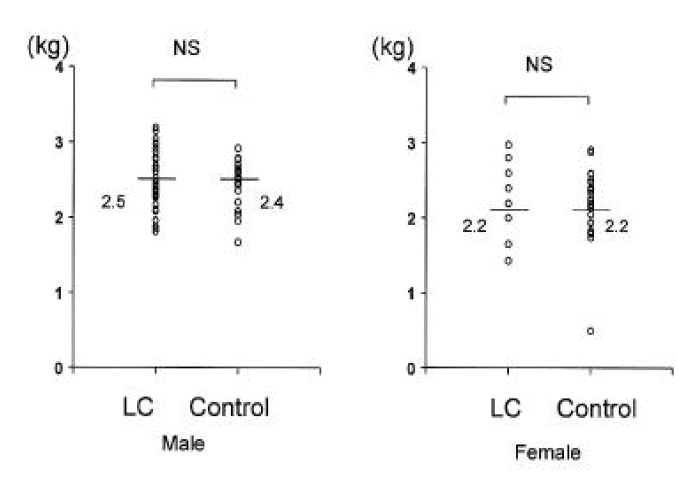
Fig. 8.
Correlation between fat mass (DEXA) and mid-arm fat area (anthropometry) in 37 cirrhotic patients and 39 controls, on which had been performed anthropometry and DEXA. MAFA, mid-arm fat area.

Fig. 9.
Correlation between lean body mass (DEXA) and mid-arm muscle area (anthropometry) in 37 cirrhotic patients and 39 controls, on which had been performed anthropometry and DEXA. MAMA, mid-arm muscle area.
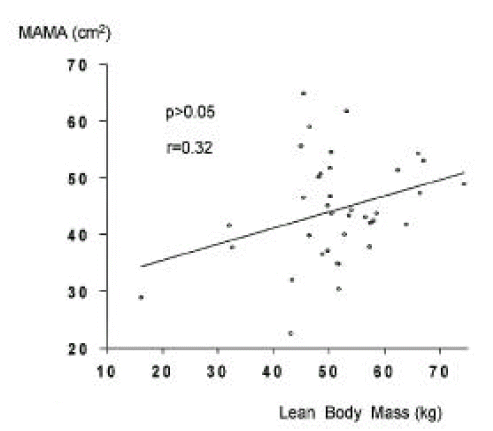
Table 1.
Characteristics of study subjects
|
Liver Cirrhosis
|
Control
|
|||
|---|---|---|---|---|
| Anthropometry | Anthropometry | |||
|
DEXA(+) (n=37) |
DEXA(−) (n=29) |
DEXA(+) (n=39) |
DEXA(−) (n=55) |
|
| Male: Female | 32: 5 | 26: 3 | 14: 25 | 35: 20 |
| Mean age (yr) | 50.6 | 53.3 | 50.2 | 54.2 |
| BMI (kg/m2) | 23.2±2.5* | 22.5±3.4 | 23.7±2.4 | 22.7±2.7 |
| Body weight (kg) | 63.9±8.8* | 61.6±11.8 | 60.4±8.6 | 56.1±7.7 |
| Child-class A | 13(35.1)** | 12(41.4) | ||
| B | 13(35.1) | 11(37.9) | ||
| C | 11(29.8) | 6(20.7) | ||
Table 2.
Anthropometric data in cirrhotic patients and controls
|
Male
|
Female
|
|||
|---|---|---|---|---|
|
Liver cirrhosis (n = 58) |
Control (n = 49) |
Liver cirrhosis (n = 8) |
Control (n = 45) |
|
| MAMA(cm2) | 43.8±9.7 | 45.9±6.6 | 32.5±7.4 | 32.9±5.6 |
| MAFA(cm2) | 11.1±7.6* | 17.9±7.4 | 13.9±7.3* | 23.9±7.9 |
Table 3.
Dual-energy X-ray absorptiometry data in patients with cirrhosis and controls
|
Male
|
Female
|
|||
|---|---|---|---|---|
|
Liver cirrhosis (n = 32) |
Control (n = 14) |
Liver cirrhosis (n = 5) |
Control (n = 25) |
|
| Fat mass (kg) | 15.4±5.6* | 21.4±4.1 | 17.2±5.9* | 23.9±4.8 |
| Lean soft tissue mass (kg) | 53.9 ±7.5 | 54.9±12.8 | 35.1±13.4 | 43.1±13.4 |
| Bone mineral content (kg) | 2.5±0.4 | 2.4±0.3 | 2.2±0.7 | 2.2±0.5 |
REFERENCES
1. McCullough AJ, Mullen KD, Smanik EJ, Tabbaa M, Szauter K. Nutritional therapy and liver disease. Gastroenterol Clin North Am 1989;18:619–643.


2. Merli M, Riggio O, Dally L, PINC. Does malnutrition affect survival in cirrhosis? Hepatology 1996;23:1041–1046.


3. Lautz HU, Selberg O, Körber J, Bürger M, Müller MJ. Protein-calorie malnutrition in liver cirrhosis. Clin Investig 1992;70:478–486.


4. Casafont F, Sanchez E, Martin L, Aguero J, Romero FP. Influence of malnutrition on the prevalence of bacterial translocation and spontaneous bacterial peritonitis in experimental cirrhosis in rats. hepatology 1997;25:1334–1337.


6. Porayko MK, Dicecco S, O’Keefe SJD. Impact of malnutrition and its therapy on liver transplantation. Sem Liver Dis 1991;11:305–314.

7. Cabre E, G-Huix F, A-Lacruz A, Esteve M, Acero D, F-Banares F, Xiol X. Effect of total enteral nutrition on the short-term outcome of severely malnourished cirrhosis. Gastroenterology 1990;98:715–720.


9. Prijatmoko D, Strauss BJG, Lambert JR, Sievert W, Stroud DB, Wahlqvist ML, Katz B, Colman J, Jones P, Korman MG. Early detection of protein depletion in alcoholic cirrhosis: role of body composition analysis. Gastroenterology 1993;105:1839–1845.


10. Crawford DHG, Shepherd RW, Halliday JW, Cooksley GWGE, Golding SD, Cheng WSC, Powell LW. Body composition in nonalcoholic cirrhosis: the effect of disease etiology and severity on nutritional compartment. Gastroenterology 1994;106:1611–1617.


11. Lukaski HC. Methods for the assessment of human body composition: traditional and new. Am J Clin Nut 1987;146:537–556.

12. Heymsfield SB, Lichtman S, Baumgartner RN, Wang J, Kamen Y, Aliprantis A, Pierson RN. Body composition of humans: comparison of two improved four-compartment models that differ in expense, technical complexity and radiation exposure. Am J Clin Nutr 1990;52:52–58.


13. Caregaro L, Alberino F, Amodio P, Merkel C, Bolognesi M, Angeli P, Gatto A. Malnutrition in alcoholic and virus related cirrhosis. Am J Clin Nutr 1996;63:602–609.


14. Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr 1981;34:2540–2545.


15. Italian multicentre cooperative project. Nutritional status in cirrhosis. J Hepatol 1994;21:317–325.


16. Campillo B, Bories PN, Leluan M, Pornin B, Devanlay M, Fouet P. Short-term changes in energy metabolism after 1 month of a regular oral diet in severly malnourished cirrhotic patients. Metabolism 1995;44:765–770.


17. Romijn JA, Endert E, Sauerwein HP. Glucose and fat metabolism during short-term starvation in cirrhosis. Gastroenterology 1991;100:731–737.


18. Kondrup J, Muller MJ. Energy and protein requirements of patients with chronic liver disease. J Hepatol 1997;27:239–247.


19. Morgan MY, Madden AM. The assessment of body composition in patients with cirrhosis. Eur J Nucl Med 1996;23:213–225.


20. Madden AM, Morgan MY. The potential role of dual-energy x-ray absorptiometry in the assessment of body composition in cirrhotic patients. Nutrition 1997;13:40–45.


21. Mccullough A, Mullen KD, Kalhan SC. Measurements of total body and extracellular water in cirrhotic patients with and without ascites. Hepatology 1991;14:1102–1111.


22. Bhatla B, Moore H, Emerson P, Keshaviah P, Prowant B, Nolph KD, Singh A. Lean body mass estimation by creatinine kinetics bioimpedance, and dual energy X-ray absorptiometry in patients on continuous ambulatory peritoneal dialysis. ASAIO Journal 1995;41:M442–M446.


23. Stenver DI, Gotfredsen A, Hilsted J, Nielsen B. Body composition in hemodialysis patients measured by dual-energy X-ray absorptiometry. Am J Nephrol 1995;15:105–110.


24. Stall S, Ginsberg NS, Lynn RI, Zabetakis PM. Bioelectrical impedance analysis and dualenergy X-ray absorptiometry to monitor nutritional status. Peritoneal Dialysis Internation 1995;15:S59–S62.

25. Crawford DHG, Cuneo RC, Shepherd RW. Pathogenesis and assessment of malnutrition in liver disease. J Gastro Hepatol 1993;8:89–94.

26. Bikle DD, Genant HK, Cann C, Recker RR, Halloran BP, Strewler GJ. Bone disease in alcohol abuse. Ann Intern Med 1995;103:42–48.

27. Diamond T, stiel D, Lunzer M, Wilkinson M, Posen S. Ethanol reduces bone formation and may cause osteoporosis. Am J Med 1989;86:282–288.


28. Reid IR, Evans MC, Ames R. Relationships between upper-arm anthropometry and soft-tissue composition in postmenopausal women. Am J Clin Nutr 1992;56:463–466.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



