INTRODUCTION
IgA nephropathy (IgAN), the most common glomerulonephritis in the world, is known to progress insidiously to end-stage renal disease in 30% to 35% of patients within 20 to 30 years1,2). To ameliorate the progression, many therapies have been tried but none has shown sufficient promise. Among them, angiotensin-converting enzyme (ACE) inhibitors are believed effective in attenuating the progressive deterioration of renal function. However, the therapeutic benefit of ACE inhibition varies among IgAN patients3).
Recently, three types of ACE gene were identified by the presence (I allele) or absence (D allele) of a 287 bp repeated sequence in intron 16 of ACE gene, and the D allele was known to be associated with higher plasma ACE activity4,5). It was hypothesized that the ACE genotype might be a determinant of the renal responsiveness to ACE inhibitors. However, the studies on the relationship between ACE I/D genotype and renal responsiveness to ACE inhibitors were conflicting and studies were and conducted on the short-term observation6).
The aims of this study were to examine the baseline characteristics of each ACE genotype in biopsy-proven IgAN patients, and to evaluate the renal responsiveness to ACE inhibitors in IgAN patients during relatively long periods according to ACE genotype.
MATERIALS AND METHODS
1. Materials
We retrospectively studied 61 biopsy-proven IgAN patients (31 men and 30 women, mean 36.3 ┬▒ 11 years, 16 to 62 years of age) with blood pressure over 130/80 mmHg or 24hr proteinuria over 1 g/day at Korea University Hospital from January 1990 through December 1997. End point of the study was the start of dialysis or March 1998, and the mean follow-up duration was 44.6 ┬▒ 26.8 months (median 44.5 months, range 5 to 113 months). All 61 patients were prescribed with ACE inhibitors (captopril and enalapril) when renal biopsy was done. Blood pressure measurements were taken using standard mercury sphygmomanometers at monthly visits and controlled below 130 / 80 mmHg during the follow-up period. Controls (N=120, 62 men and 58 female, age 46.9 years range: 16 to 62 years) were chosen from healthy volunteers with normal blood pressure, BUN, serum creatinine and urinanalysis.
2. Methods
We obtained information on sex, age, duration of disease, hypertension (> 140/90mmHg), proteinuria (initial and follow-up) serum creatinine (initial and follow-up), and azotemia (initial and follow up: serum creatinine > 1.4 mg/dL). ŌĆ£InitialŌĆØ means before prescription of ACE inhibitors and ŌĆ£follow-upŌĆØ means the end point of study after prescription of ACE inhibitors. The renal responsiveness to ACE inhibitors was determined by the degree of changes of proteinuria at 12 months after prescription of ACE inhibitors and by the slope of reciprocal variation of the serum creatinine during follow-up duration {(1/Cr2 - 1/Cr1)/durations}.
To determine ACE genotype, we obtained venous blood samples and extracted genomic DNA from leukocytes in buffy coat using QIAamp Blood Kit (Qiagen, Germany). A 287-bp I/D polymorphism in the intron 16 of the ACE gene was examined by polymerase chain reaction(PCR). The sequences of the sense and antisense primers were 5ŌĆ▓-CTG GAG ACC ACT CTT TCT-3ŌĆ▓ and 5ŌĆ▓-GAT GTG GCC ATC ACA TTC GTC AGA-3ŌĆ▓, respectively (Genosys DNA synthesizer, USA). PCR was performed in a final volume of 50 ╬╝L that contained 50 ng of genomic DNA, 40 pmol of each primer, 125 ╬╝mol each of the four dNTP, 3 mM MgCl2, 50 mmol KCl, 10 mmol Tris-HCl (pH 8.3) and 1 U of Taq polymerase (Boehringer Mannheim, Germany). Amplification was carried out for 30 cycles with steps of denaturation at 94┬░C for 1 min, annealing at 54┬░C for 1 min and extension at 72┬░C for 1 min. The PCR products were subjected to electrophoresis in 1% agarose gels. Amplication of the D allele resulted in a 190 bp DNA fragment and amplification of the I allele resulted in a 490 bp fragment. Homozygotes had a single 190 (DD) or 490 (ID) bp band; heterozygotes had one 190 bp and one 490 bp band.
To verify DD genotype, the PCR products of all DD genotypes were examined by 2nd PCR. The sequences of the sense and antisense primers from insertion specific sequence were 5ŌĆ▓-TGG GAC CAC AGC GCC CGC TAC-3ŌĆ▓ and 5ŌĆ▓-TCG CCA CTC CCA TGC CCA TAA-3ŌĆ▓, respectively (Genosys DNA synthesizer, USA). PCR was performed in a final volume of 50 ╬╝L that contained 50 ng of genomic DNA, 40 pmol of each primer, 125 ╬╝mol each of the four dNTP, 3 mM MgCl2, 50 mmol KCl, 10 mmol Tris-HCl (pH 8.3) and 0.5 U of Taq polymerase (Boehringer Mannheim, Germany). Amplification was carried out for 30 cycles with steps of denaturation at 94┬░C for 30 sec, annealing at 67┬░C for 45 sec and extension at 72┬░C for 2 min. The PCR products were subjected to electrophoresis in 1% agarose gels. Random replication of PCR amplification was used to verify all of the results.
First, we examined the baseline characteristics according to genotype. Second, we evaluated the renal responsiveness to ACE inhibitors, and compared the above variables among three genotypes.
RESULTS
The distribution of II, ID and DD genotypes in controls was 25, 63 and 32, respectively.
Out of 61 patients, the distribution of the II, ID and DD genotypes was 21, 16 and 24 patients, respectively. There were no differences among the three genotypes in baseline characteristics such as sex, age, initial serum creatinine level and initial 24-hrs proteinuria, and the number of patients whose initial blood pressure were over 140/90 mmHg, initial serum creatinine > 1.4 mg/dl, and initial proteinuria > 2.0g/day (Table 1).
Mean follow-up duration of each genotype was II genotype, 36.6 ┬▒ 22.9 months (median 30.0 months, range 6 to 79 months), ID genotype, 49.3 ┬▒ 31.2 months (median 43.0 months, range 5 to 113 months), and DD genotype, 48.1 ┬▒ 26.3 months (median 48.5 months, range 10 to 108 months) (Table 1).
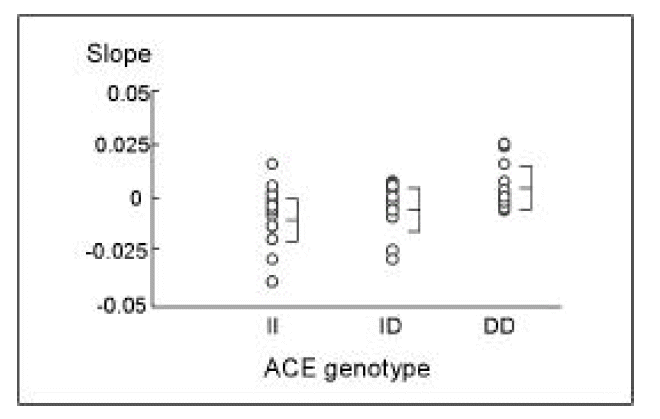
There were no differences among the three genotypes in the follow-up serum creatinine level and the number of patients with development of azotemia(follow-up serum creatinine > 1.4 mg/dl) during follow-up periods (Table 1). However, significant changes were noticed in the slopes of reciprocal variation of the serum creatinine against follow up duration {(1/Cr2-1/Cr1/durations} (Fig. 1). The mean slopes according to three genotypes were ŌłÆ0.0059 ┬▒ 0.0130 dŌäō *mgŌłÆ1*monthŌłÆ1 in II, 0.0038 ┬▒ 0.0109 dŌäō*mgŌłÆ1*monthŌłÆ1 in ID, and 0.0187 ┬▒ 0.0008 dŌäō*mgŌłÆ1*monthŌłÆ1in DD (Table 1). A significant difference was noticed between II and DD genotype (p= 0.05). However no significant differences were shown between ID and DD genotype (p= 0.23), and between II and ID genotype (p= 0.83). Therefore, ACE inhibitors in DD genotype of IgAN were more effective than in II genotype and ID genotype in attenuating the progression of renal disease.
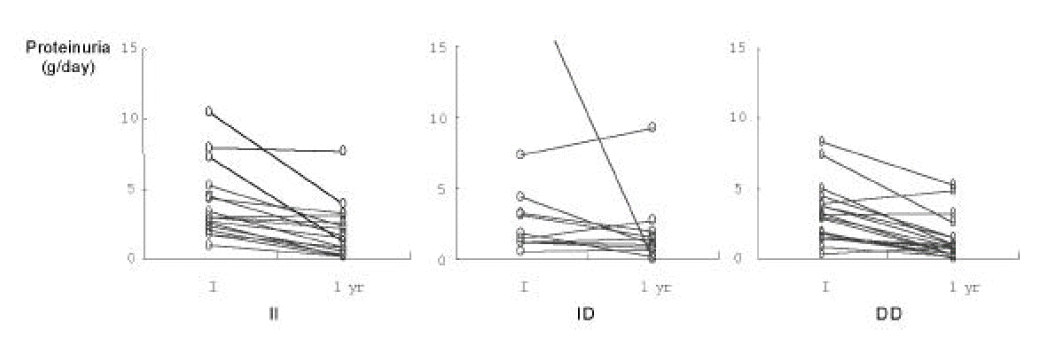
There were no significant differences among three genotypes in the patients with initial proeinuria over 2.0 g/day(Table 1) and over 3.5 g/day (II: 8, ID: 5, DD: 9). Also, there were no significant differences among three genotypes in the degree of changes of proteinuria at 12 months after ACE inhibitor prescription. However, antiproteinuric responsiveness to ACE inhibitors at 12 months after prescription was noticed significantly in most of the IgAN patients regardless of genotypes (initial 3549.1 ┬▒ 3259 mg, median: 3000 mg; after 12 months 1672.9 ┬▒ 1879 mg, median: 985 mg; p < 0.001, Fig. 2).
DISCUSSION
We showed that ACE inhibitorsŌĆÖ efficacy on renal function preservation in IgAN was more pronounced in DD genotype than II genotype when we compared the slopes of reciprocal variation of the serum creatinine against follow-up duration. Also, the significant antiproteinuric response to ACE inhibitors was found in IgAN, but no significant difference was found among three ACE genotypes.
Because of the relatively long observation period (mean 44.6, median 44.5 months, range 5 to 113 months) of this study, we expected that the long-term renal protective effects of ACE inhibitors in IgAN would be variable according to ACE gene polymorphism. However, with regard to the antiproteinuric responsiveness, we could not find a significant difference among the three genotypes. This suggests that other mechanisms by ACE inhibitor besides antiproteinuric effect may contribute in preserving the renal function in IgAN.
It has been reported that the distribution of ACE genotypes in IgAN is similar to that in the general population7,8,9). The association between DD genotype and the renal disease progression was controversial. Some reported that the genotypes with D allele were not related to the progression of glomerulonephritis including IgAN9,10). On the other hand, others reported the progression of IgAN may be influenced by the genotypes with D allele8,11,12). Dissimilar to the above studies which observed the natural course of IgAN, we observed the course of IgAN after therapeutic intervention with ACE inhibitors.
ACE plays a key enzyme in the renin-angiotensin and kallikrein-kinin system by activating angiotensin I into angiotensin II and by inactivating bradykinin13,14,15). The renin-angiotensin system is believed to play an important pathophysiologic role in the progression of chronic renal disease. ACE inhibitors have been reported to attenuate the progression of chronic renal disease such as primary glomerulonephritis or diabetic nephropathy16,17,18).
An ACE gene polymorphism has been known as an important genetic factor influencing the plasma and cellular ACE levels; ACE activity is known to be higher in the order of DD, ID, II4,5). Therefore, activities of local angiotensin II and bradykinin may be related to ACE gene polymorphism. Probably because II genotype was associated with lower angiotensin II level in the kidney than DD genotype, ACE inhibition in II genotype may be less efficient on renal function preservation compared with that in DD genotype11). We also found that ACE inhibitors were more efficient in DD genotype in preserving renal function in IgAN when comparing the slope of creatinine variation against follow-up duration. In comparison to other studies, we observed relatively longer periods(median 44.5 months, range 5 to 113 months). We observed the course of six IgA patients for less than one year; the distribution of II, ID and DD genotypes was 3, 1, 2, respectively. However, because of small sample size, a future large-scale study should be done to generalize and confirm our positive findings.
Antiproteinuric effect of ACE inhibitors was firstly reported by de Jong et al19). Some reported ACE inhibitors were more effective in antiproteinuric effect than any other antihypertensive drugs20,21). Also, some reported antiproteinuric effects of ACE inhibitors were more pronounced in DD genotype than II or ID genotype of IgAN patients at 1 year after prescription of ACE inhibitors6,11,12). However, we found that antiproteinuric effect of ACE inhibitors in IgAN was not different among the three genotypes. This discrepancy may be related to the small sample size of this study and the abrupt antiproteinuric response to ACE inhibitors in a few patients with II and ID genotype.
Antiproteinuric effect of ACE inhibition is now widely accepted through the hemodynamic effect of ACE inhibitor besides reducing systemic blood pressure. These changes in renal hemodynamics are mostly likely due to the reduced local angitensin II formation and the augmented local bradykinin formation in the kidney, which elicit decreased efferent arteriolar resistance and subsequent fall in intraglomerular pressure15,19).
Another mechanism, besides antiproteinuric effect may be present in renal function preservation of IgAN by ACE inhibition. Angiotensin II may act as a growth factor on several renal cells, including mesangial cells and tubular epithelial cells, besides constricting action on vascular smooth muscle cells22,23). Angiotensin II has been known to promote the growth changes and the synthesis of extracellular matrix protein through stimulating the release of transforming growth factor-╬▓ (TGF- ╬▓)24,25). Therefore angiotensin II is regarded as having a pathophysiologic role in renal disease progression.
In conclusion, our results suggest that ACE inhibitorsŌĆÖ efficacy on antiproteinuric action is significant in IgAN, and that ACE inhibitorsŌĆÖ efficacy on renal function preservation in IgAN is more pronounced in DD genotype than II genotype.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





