 |
 |
| Korean J Intern Med > Volume 16(3); 2001 > Article |
|
Abstract
Background :
It has been widely accepted that the epitope (s) and/or functional characteristics of thyrotropin receptor antibodies (TSHRAb) from Graves’ patients are heterogenous among patients. However, the clinical significance of such heterogeneity has not been systematically evaluated yet. We were to elucidate and find the clinical significance of heterogeneity for TSH receptor antibodies in Graves’ disease.
Methods :
We measured stimulating TSHRAb (TSAb) activities using CHO-hTSHR cells, FRTL-5 cells and chimeric receptor expressing cells (Mcl+2 and Mc2), specific blocking TSHRAb (TSBAb) activities using Mc2 cells and TBII activities using porcine thyroid membrane in 136 patients with untreated hyperthyroid Graves’ disease.
Results :
Based on various TSHRAb activities from each patient, the patients could be categorized into 7 subgroups by cluster analysis: 1) Group 1 (n=41) was characterized by moderate TSAb activities both in CHO-hTSHR cells and in FRTL-5 cells, typical TSAb epitope, rare blocking antibodies and high TBII activities. 2) Group 2 (n=16) was characterized by the presence of blocking TSHRAb in most patients, albeit the other characteristics were the same as those in Group 1. 3) Group 3 (n=19) patients had low TSAb activities both in CHO-hTSHR cells and in FRTL-5 cells, seldom had blocking TSHRAb, but they had high TBII activities. 4) Group 4 (n = 30) could be categorized as ‘mild disease’ group, as they had low activities in all kinds of TSHRAb assay and had low antimicrosomal antibody activities. 5) Group 5 (n=14) was characterized by moderate TSAb activities with atypical epitope (s), rare blocking TSHRAb and moderate TBII activities. 6) Group 6 (n=10) patients had very high TSAb activities with typical epitopes, seldom blocking TSHRAb and low TBII activities. 7) Group 7 (n = 6) was characterized by very high TSAb activities with atypical epitopes and high TBII activities. Pretreatment serum thyroid hormone level was low only in group 4 patients compared to the other 6 groups (p<0.05). The size of goiter was significantly larger in those in group 1 and group 3 (p<0.05) compared to the other 5 groups. The prevalence of clinically significant ophthalmopathy was higher in group 2 patients than the other 6 groups (50% vs. 27.5%, p=0.06). Among 6 kinds of TSHRAb activities, only the blocking TSHRAb activity was significantly associated with the presence of ophthalmopathy in multivariate analysis.
Conclusion :
These results suggest that the differences in epitopes for TSAb or the presence of blocking TSHRAb is not a major factor in determining the degree of thyrotoxicosis in Graves’ disease. Although the pathogenic mechanism is not clear yet, we suggest that patients with ophthalmopathy have different TSHRAb repertoire from those without ophthalmopathy in Graves’ disease.
Epitopes for stimulating TSH receptor antibodies (TSHRAb) are well known to be heterogenous among patients with Graves’ disease1–7). We recently reported that such heterogeneity may be linked to the differences in their responses to antithyroid drug treatment6). TSHRAb from Graves’ patients are heterogenous among patients not only in epitope recognition but also in functional characteristics8, 9). If such differences in functional characteristics are related to epitope heterogeneity, it is not clear yet. Moreover, some clones of peripheral lymphocyte from patients with hyperthyroid Graves’ disease can produce blocking TSHRAb10, 11), but the clinical relevance or the prevalence of such blocking antibodies in those patients are not known.
To define the clinical implication of such heterogeneity, we need a large cohort of patients and suitable assay methods for dissecting the antibody characteristics among patients. As we recently reported6), stimulating TSHRAb activity measured in FRTL-5 cells and those measured in human TSHR transfectant (CHO-hTSHR) may not represent identical populations of stimulating antibodies. Thus, patterns of receptor stimulation by antibodies in two cell systems may be one of the tools to characterize the stimulating TSHRAb which patients have. Moreover, stable transfectants expressing human TSH receptor - rat LH/CG receptor chimera, Mc 1+2 and Mc 2, were useful assay systems defining epitope heterogeneity of stimulating TSHRAb of Graves’ patients6, 12).
Mc 2 cells, in which 90th to 165th a.a. residues of extracellular domain of TSHR were replaced, have good cyclic AMP responses to TSH but poorly stimulated by most IgGs from Graves’ patients6). So, it seemed to be a good candidate assay system for measuring blocking TSHRAb activities with Graves’ sera. Using this cell, we show here that significant numbers of patients with hyperthyroid Graves’ disease have blocking TSHRAb activities.
To elucidate the clinical significance of heterogeneity of TSHRAb in Graves’ disease, we measured stimulating TSHRAb activities with FRTL-5 cells, CHO-hTSHR cells, Mc1+2, and Mc 2 cells, blocking TSHRAb activities with Mc 2 cells, and TBII activities in 136 untreated patients with Graves’ disease. Based upon 6 kinds of various TSHRAb activities, patients were classified using cluster analysis and clinical characteristics were compared in each of the subgroups classified.
The cAMP RIA kits were obtained from Instars (Minnesota, MN), protein A-Sepharose CL-4B columns were purchased from Pharmacia Fine Chemicals (Uppsala, Sweden) and Coon’s Modified Ham’s F-12 medium was obtained from JRH Biosciences (Lenexa, KN). F-12 nutrient mixture, G418 (Geneticin), antibiotic-antimycotic solutions, glutamine, trypsin-ethylenediamine tetraacetate solutions, fetal calf serum, lipofectin® and OPTI-MEM1® were from Life Technologies (Grand Island, NY). Polyethylene glycol (PEG, M.W. 4000), 3-Isobutyl-1-methylxanthine, BSA and bovine TSH (bTSH) were purchased from Sigma Chemical Co. (St Louis, MO). All other chemicals or materials were the highest grade available13, 14).
Sera were obtained from 33 normal controls and 136 patients with untreated hyperthyroid Graves’ disease. The diagnosis of Graves’ disease was based on clinical and laboratory criteria, including elevated serum thyroid hormone levels, undetectable TSH by sensitive RIA and diffuse goiter at scintiscan. Sera were obtained under institution guidelines and with appropriate approval and consent.
IgGs for measurements of blocking TSHRAb activities were affinity purified using Protein A-Sepharose CL-4B column, then dialyzed against 100 vol distilled water every 8 h for 2 days at 4C. After removal of denatured protein by centrifugation (1500 × g for 15 min at 4C), the IgG was lyophilized and stored at −20C until assay. Pooled normal IgG was obtained from sera of 33 normal healthy subjects with no thyroid disease.
PEG-precipitated crude immunoglobulin fraction of sera were used in the measurement of stimulating TSHRAb activities. One vol. of serum was mixed with three vol. of 20% PEG 4000 solution, then precipitated by centrifugation (2800 g for 20 min.). PEG precipitation was done immediately before assay from stored sera and a precipitate of 0.25 mL of sera was used in each assay.
hTSHR cloning, amplification of rat LH/CGR cDNA fragment and construction of chimeric receptors were done as previously described13). The chimeric receptors used in this report are designated to indicate the substituted segment of the hTSHR, numbered from methinine start site. In Mc1+2, residues 8–165 of the hTSHR were replaced by residues 10–166 of the rat LH/CGR: in Mc2, residue 90–165 of the hTSHR were replaced by residues 91–166 of the rat LH/CGR.
Stable CHO cell transfectants were made by cotransfection of the wild-type hTSHR or hTSHR-LH/CGR chimeras in pSG5 with pMAM-neo, the selection marker, using lipofectin as previously described13). Surviving cells, in Ham’s F-12 medium containing 10% FCS and 1 mg/mL geneticin were expanded for 1–2 weeks and cloned by limiting dilution. The most responsive clones to bTSH, as determined by the cAMP response, were selected and expanded. For TSHRAb assay, cells were plated in 24-well plates (3–4×104 cells/well), fed fresh medium 48 h later, and used when they were at 100% confluency, usually 60–72 h after plating. In a typical assay, there were 5–6×105 cells/well. The cAMP response to a standard amount of bTSH or a standard pool of Graves’ IgG was stable for over 3 months of continuous culture (about 30 passages).
FRTL-5 cells (ATCC CRL #8305) were grown, as previously described15), in Coon’s modified Ham’s F-12 medium containing 5% calf serum and a six-hormone mixture, including bTSH (10 U/L), insulin (10 mg/L), hydrocortisone (1 nmol/L), human transferrin (5 mg/L), somatostatin (10 μg/L), and glycyl-L—histidyl-L-lysine (10 μg/L). Cells were fed every third day and passaged every 6–9 days. For assays, FRTL-5 cells reaching near confluency in 24-well plates were incubated with medium lacking bTSH (5H media) and maintained therein for 7 days with feeding every 3 days.
Assays in CHO cells were routinely performed in NaCl-free Hank’s Balanced Salt Solution (HBSS) [5.4 mM KCl, 1.3 mM CaCl2, 0.8 mM MgSO4, 0.3 mM Na2HPO4, 0.4 mM KH2PO4, 0.1% glucose] containing 20 mM HEPES (pH 7.4), 1% BSA, 0.5 mM 3-isobutyl-1-methylxanthine, and 222 mmol/L sucrose to make it isotonic. Assays in FRTL-5 cells were performed in the same medium without sucrose. Purified IgG (10 g/L) or PEG-precipitated sera (0.25 mL of sera equivalent / well) were dissolved in incubation media to the desired concentration and were added to the cell. Incubations were performed for 2 h at 37 C before the incubation medium was aspirated and frozen at −20 C. The cAMP released into the medium was measured by RIA after dilution with sodium acetate buffer according to the manufacturer’s instruction. All samples were run in duplicate or triplicate. Stimulating TSHRAb activity is expressed as the percent increase in cAMP production with the test IgG by comparison to assays with the equivalent amount of pooled normal IgG. Stimulating TSHRAb activity was defined as positive when the value was greater than 2 SD above the mean value of IgG from 33 normal subjects.
In assays for blocking TSHRAb activities, Mc 2 cell was used as this cell is well stimulated by bTSH but not stimulated by Graves’ IgGs from most patients. For blocking TSHRAb assay, we routinely used the affinity purified IgGs. The percent inhibition of cAMP production stimulated with bTSH (0.1 U/L) by test IgG (5 g/L) by comparison to pooled normal IgG was expressed as blocking activity. Blocking TSHRAb activity was defined as positive when the value was greater than 2 SD above the mean value of IgG from 33 normal subjects.
Serum free T4 and total T3 concentrations were measured using commercial kit (Abbott, North Chicago, IL); the normal ranges were 0.9–2.1 and 85–178 ng/dL, respectively. Serum TSH was measured by immunoradiomatric assay (Abbott); the normal range was 0.38–4.1 mU/L. Serum antithyroglobulin and antithyroid peroxidase antibodies were measured with commercial kits (R.S.R., Cardiff, UK); a titer of greater than 0.3 U/mL was considered positive. Thyrotropin binding inhibitor immunoglobulin (TBII) activity was measured with radioreceptor assay kit (R.S.R.) as previously described (16). TBII activity is expressed as the percent inhibition of [125I] TSH binding: a TBII activity exceeding 15%, which is 2 S.D. above the mean value from 64 normal samples, was considered positive. The intraassay and interassay variance of TBII activity were 1.7–8.0% and 3.7–10.5%, respectively.
The data is expressed as the mean ± S.D. Statistical analysis for comparing values of two groups was done with student’s t test or with Wilcoxon rank sum test. To determine significant difference in multiple comparisons, we used Kruskal-Wallis one-way ANOVA. Duncan’s multiple range test was performed when the ANOVA indicated a significant difference. Differences in categorical data between subgroups were analyzed by qui square test with Yates’ correction (two-tailed). Multiple linear logistic regression analysis were used for identifying variables that predict the presence of ophthalmopathy.
Cluster analysis was used to classify the patients into subgroups based on their various TSHRAb activities. After standardization of variables, the distance between variables was measured by squared Euclidian distance, and the method of clustering was average linkage. All the statistical analysis was performed by SPSS PC program. p<0.05 was considered statistically significant.
One hundred and thirty six consecutive patients with untreated hyperthyroid Graves’ disease were enrolled. Clinical characteristics and initial laboratory findings are shown in Table 1. Twenty two patients (16.2%) had previous history of Graves’ disease, but any patient who had ever taken antithyroid drugs during the recent 6 months period were excluded from the study. Clinically significant signs of ophthalmopathy were found in 41 patients (32.5%).
Stimulating TSHRAb activities of 136 patients with Graves’ disease were measured by wild-type CHO-hTSHR cells and by FRTL-5 cells. Stimulating TSHRAb activities were positive in 81.6% (111/136) and 92.7% (126/136) of patients when measured by CHO-hTSHR cells and by FRTL-5 cells, respectively. The mean activities measured by CHO-hTSHR cells were lower than those measured by FRTL-5 cells (481 ± 573% vs. 1025 ± 968%, p<0.05). The two stimulating Ab activities correlated significantly with each other (R=0.53, p<0.01) however, there were marked discrepancies between the two in some patients (Figure 1).
The stimulating TSHRAb activities of IgGs from patients measured by CHO-hTSHR-LH/CGR chimera, Mc1+2, in which 8–165th amino acid residues are replaced by those of rat LH/CG receptor were positive in 16.9% (23/136) of patients (Figure 2., left column). In Mc 2, in which 90–165th amino acid residues are replaced, stimulating TSHRAb activities were positive in 13.2% (18/136) of patients (Figure 2., right column). The stimulating TSHRAb activities measured by the chimeric receptors were much lower than those measured by wild-type hTSHR in most patients, showing similar results to our previous report6). 71.3% of total patients (97/136) were negative both in Mc1+2 and in Mc 2 assay. The stimulating TSHRAb activities measured by Mc1+2 were not correlated with those measured by Mc2 (Data not shown).
Even if there are TSHRAb with blocking activities in sera of some hyperthyroid patients with Graves’ disease, there are limitations in accurate measurements of blocking TSHRAb activities with wild-type TSH receptors. A major impediment for this is the co-stimulation of the receptor by stimulating TSHRAb existing in most Graves’ sera in addition to bTSH added in blocking TSHRAb assay. And, moreover, the degree of co-stimulation varies from patient to patient.
As we previously reported6) and as we have shown in this paper, epitope(s) for stimulating TSHRAb from most patients with untreated Graves’ disease are located in 90–165th a.a. region of TSH receptor. So, we expected that Mc 2 chimera could detect blocking TSHRAb more efficiently than wild-type CHO-hTSHR because Mc 2 could avoid the receptor stimulation caused by stimulating antibodies. The cAMP response to bTSH and to pooled IgG from patients with active Graves’ disease supported this possibility. As shown in Figure 3., Mc 2 cells are well stimulated by bTSH showing that cAMP responses were consistently over 10 fold of basal, but not stimulated by Graves’ IgG significantly.
So, the blocking TSHRAb activities of IgGs from Graves’ patients were measured using CHO-hTSHR-LH/CGR chimeras, Mc 2. In 17.6% of patients (24/136) with hyperthyroid Graves’ disease, blocking TSHRAb were detected (Figure 4). Those having blocking antibodies had significantly lower mean stimulating TSHAb activities measured by wild-type CHO-hTSHR cells than those without blocking antibodies (309 ± 182 vs. 518 ± 621%, p<0.05) and tended to have higher TBII activities (52.3 ± 26.5 vs. 43.0 ± 22.5%, p=0.06) (Table 2).
The patients with blocking antibodies were not different from those without blocking antibodies in age, sex ratio, degree of thyrotoxicosis and goiter size. However, unexpectedly, the prevalence of clinically significant ophthalmopathy tended to be higher in those patients with blocking antibodies (46.8% vs. 26.8%, p=0.06) (Table 3).
Among 6 kinds of various TSHRAb activities measured, only the blocking TSHRAb activity was significantly associated with the presence of ophthalmopathy in multiple linear logistic regression analysis (p<0.05, Table 4).
Cluster analysis was done setting the 6 kinds of TSHRAb activities measured as independent variables to classify the patients with Graves’ disease according to the characteristics of TSHRAb they have. Patients could be classified into 7 subgroups in minimum, and mean activities of each TSHRAb activities in subgroups classified are shown in Table 5. Simplifying the results, the characteristics of TSHRAb in each subgroups are summarized in Table 6. “Typical TSAb epitope” means no or infrequent presence of stimulating TSHRAb activities measured by CHO-hTSHR-LH/CGR chimera, Mc1+2 or Mc 2.
Group 1 (n=41) was characterized by moderate TSAb activities both in CHO-hTSHR cells and in FRTL-5 cells, typical TSAb epitope, rare blocking antibodies and high TBII activities. This group included the largest numbers of patients and seemed to be the typical TSHRAb of Graves’ disease. Group 2 (n=16) was characterized by the presence of blocking TSHRAb in most patients, albeit the other characteristics were the same as those in Group 1. Group 3 (n=19) patients had low TSAb activities both in CHO-hTSHR cells and in FRTL-5 cells and seldom had blocking TSHRAb, but they had high TBII activities. Group 4 (n=30) could be categorized as ‘mild disease’ group, as they had low activities in all kinds of TSHRAb assay and had low antimicrosomal antibody activities. Group 5 (n=14) was characterized by moderate TSAb activities with atypical epitope(s), rare blocking TSHRAb and moderate TBII activities. Group 6 (n=10) patients had very high TSAb activities with typical epitopes, seldom blocking TSHRAb and low TBII activities. Group 7 (n=6) was characterized by very high TSAb activities with atypical epitopes and high TBII activities. The presence of blocking TSHRAb could not be determined in this group because of very high TSAb activities for Mc2 cell.
The clinical characteristics of patients in the 7 groups were compared (Table 7). There were no differences in age, sex ratio, fraction of recurred cases, numbers of patients with family history of thyroid diseases, mean serum free T4 concentration and thyroglobulin antibody activities among the groups. Prevalence of clinically significant ophthalmopathy tended to be higher in group 2 patients compared to the patients in the other groups (50.0% vs. 27.5%, p=0.06, Table 7). Goiter size was significantly larger in group 1 and in group 3 patients compared to the other groups (p<0.05 in ANOVA, Table 7). Pretreatment mean serum T3 concentration and antimicrosomal antibody titer was significantly lower in group 4 patients compared to the other (p<0.05 in ANOVA, Table 7).
TBII activities were significantly correlated with goiter size (R=0.38, p<0.05) or with serum T3 concentrations (R=0.37, p<0.05) in group 1 patients (Figure 5). Also, in group 2 patients, TBII activities had significant correlation with goiter size (R=0.52, p<0.05) or with serum T3 concentrations (R=0.48, p<0.05) (Figure 6). However, there was no significant correlation between TBII activities and clinical parameters in patients of other groups (Table 8). Thus, we found that TBII activities reflect the disease activity in terms of degree of thyrotoxicosis or of goiter size only in a subset of patients, about 40% of total, with Graves’ disease. In group 3 patients, the antimicrosomal antibody activities were significantly correlated with serum free T4 levels (R=0.49, p<0.05) or with goiter size (R=0.36, p=0.06) (Figure 7).
In this report, we have tried to classify the patients according to the characteristics of TSH receptor antibodies they have and to observe if such characteristics are associated with different clinical manifestations of those patients. It is based on observations that the TSH receptor antibodies of Graves’ sera are mixtures of antibodies with different epitopes and functional characteristics1–11). Using the stable transfectants expressing mutant TSH receptor (Mc 2), we firstly documented that blocking TSHRAbs are found in significant numbers of patients with untreated hyperthyroid Graves’ disease (18%) and that it is associated with the presence of ophthalmopathy.
In dissecting the epitopic and functional characteristics of TSHRAb found in Graves’ sera, we used the TSH receptors from different species (rat and human TSH receptors), two different hTSHR-rLH/CG chimeric receptors (Mc 1+2 and Mc 2) and checked the receptor binding affinity of antibodies to porcine thyroid membrane (TBII). And, more importantly, we measured relatively specific blocking TSHRAb activities using chimeric receptor expressing cell, Mc 2. We have recently reported that TSAb assay with rat TSH receptor and that with human TSH receptor may detect different functional populations of TSHRAbs6). Recently Patibandla et al17) found that antibodies in Graves’ sera bind to human TSH receptor or to mouse TSH receptor in a much different fashion suggesting that the minor differences in amino acid sequence could result in great disparities between receptor structures. So, it is assumed that patterns of stimulation of rat TSH receptor and/or of human TSH receptor could be one of the tools to define epitopic characteristic of receptor antibodies. The chimeric receptor transected cells, Mc 2 and Mc1+2 we used here, are useful cell lines to detect stimulating TSHRAbs involving epitopes on the N-terminal portion of the TSHR extracellular domain, as we reported6). Moreover, as the stimulating TSHRAb and TBII epitope in Graves’ disease may be different10, 11, 18), presence or absence of TBII activities and/or the activity itself may be another characteristic associated with epitopic reactivity of TSHRAb. Using six different assays for TSHRAb, we have tried to categorize the TSHRAb of Graves’ disease to define epitope heterogeneity.
One of impediments to measure six kinds of TSHRAb activities in a large number of patients was the great time and effort required in IgG preparation. So we checked if the crude IgG fraction prepared by PEG precipitation of sera could be used in TSHRAb assays. High correlation coefficient and high concordance rates between crude IgG fraction and affinity purified IgG in stimulating TSHRAb assay suggested that crude IgG fraction could be used instead of affinity purified IgG. So, all the stimulating TSHRAb assay was done with PEG precipitated sera in this study. However, in blocking TSHRAb assay, use of crude IgG fraction decreased the detection rate significantly when compared to assays using affinity purified IgG. The exact mechanism for this is not clear. One of possibilities is that of nonspecific interaction between bTSH used in blocking TSHRAb assay and proteins in precipitated sera hindering bTSH from stimulating the receptor. The other possibility is that the nonspecific receptor blocking activities of precipitated control sera could raise the positive cut-off values for blocking TSHRAb activities. Based on these observations, the blocking TSHRAb activities were measured with affinity purified IgG using protein A column.
Stimulating TSHRAb activities measured by FRTL-5 cells were roughly correlated with those by CHO-hTSHR cells, but there were marked discrepancies between the two in some patients. This seems to represent the epitope heterogeneity for stimulating TSHRAb among the patients with Graves’ disease. In CHO-hTSHR-LH/CGR chimeras assays, stimulating TSHRAb activity was found in 16.9% and 13.3% of patients in Mc1+2 and Mc 2 cells, respectively, showing similar results to our previous study in a totally different set of patients6, 12). This figure is also similar to that of Japanene cohort of Graves’ disease19) in which stimulating TSHRAbs in Mc 2 cells were detected in 23% of patients. These reconfirm that the major functional stimulating TSHRAb epitope lies on 90–165th a.a. residues of extracellular domain of TSHR. For this finding, the TSHRAbs having no stimulating activities in both chimera were considered to have “atypical” TSAb epitope.
The major and the most important finding in this study is that blocking TSHRAb activities are found in significant numbers, in 18%, of patients with untreated hyperthyroid Graves’ disease, and that the presence of blocking antibodies is associated with clinically significant ophthalmopathy. One of the reasons for higher prevalence of blocking TSHRAb in this study than previously noted20) might be the use of Mc 2 cell. It could avoid receptor stimulation by coexisting stimulating antibodies of most patients because the most functionally important TSAb epitope region was replaced, whereas it retained the reactivity to TSH in similar degrees to that in wild-type TSHR6, 13). As it is not feasible to separate antibodies with blocking activity from stimulating antibodies, such strategy for detecting blocking TSHRAb in Graves’ sera is essential. In a recent study using same Mc 2 cell as this study, blocking TSHRAb has not been found in 24 patients with Graves’ disease19), in which PEG precipitated sera were used for blocking TSHRAb assay. As described above, it is important to use purified IgG to increase the detection rate for blocking TSHRAb. This might be another reason to explain the higher prevalence of blocking antibodies in Graves’ patients in this study.
There were no differences in the degree of thyrotoxicosis or in goiter size between those with blocking antibodies and those without. Although the blocking TSHRAbs are found in significant numbers of untreated hyperthyroid patients with Graves’ disease, it seems not to be potent in its activity to affect the clinical course.
The association of blocking TSHRAb with ophthalmopathy in Graves’ disease is a new and interesting finding. The prevalence of ophthalmopathy was higher in group 2 patients of our study characterized by the presence of blocking TSHRAb activities in most patients than patients of other groups classified. The result of multivariate analysis for the presence of ophthalmopathy showed that the blocking TSHRAb activity was the only TSHRAb activity significantly associated with ophthalmopathy among various TSHRAb activities we measured. This study is the first to find the association of blocking TSHRAb with ophthalmopathy in Graves’ disease. Although the exact pathophysiological basis for this is not clear at this point, there are two possibilities.
Firstly, the association of blocking TSHRAb with ophthalmopathy is a epiphenomenon reflecting the occurrence of more severe degrees of autoimmune responses to TSHR found in patients with ophthalmopathy than those without ophthalmopathy. The higher prevalence of TSHRAb positivity, measured by TBII or TSAb activities, in those with ophthalmopathy than those without supports this possibility21–26). If patients with ophthalmopathy have higher titers of various kinds of autoantibodies reactive with the TSHR, not only stimulating but also blocking antibodies, the higher prevalence of blocking antibodies in those patients may be merely a marker of severe immunologic pertubations found in them.
Secondly, Graves’ patients with ophthalmopathy may have qualitatively different TSHRAbs from those without ophthalmopathy. The fact that the clinical course of hyperthyroidism may be irrelevant to that of ophthalmopathy in Graves’ disease27) and that thyroid-associated ophthalmopathy can occur in euthyoid subjects28) suggest that the stimulating TSHRAb itself is not the cause of ophthalmopathy. The frequent de novo development or transient increase in activities of blocking TSHRAb after radioiodine treatment of Graves’ disease29) and frequent aggravations of ophthalmopathy after raidioiodine treatment30) suggest the association of blocking TSHRAb in the development of ophthalmopathy. More interestingly, some have found the association of post-RAI Tx. aggravation of ophthalmopathies with development of hypothyroidism in Graves’ patients31, 32). Observation of patients with Graves’ disease before and after radioiodine treatment with sensitive diagnostic imaging, such as CT or MRI scan of orbit and concurrent specific measurements of blocking TSHRAb activities by methods using Mc 2 cells, will clarify this point. Epitopes for blocking TSHRAb of autoimmune thyroid disease is known to be located in the C-terminal portion of the extracellular domain of TSHR33). Burch et al34) reported that the antisera directed against immunogenic domain of hTSHR (a.a. 352–367) specifically bind to cultured retroocular fibroblast obtained from Graves’ patients with ophthalmopathy. These findings suggest that the autoimmune reaction to the C-terminal portion of TSHR is important in the development of ophthalmopathy. Peptide library screen using C-terminal peptides in Graves’ patients with or without ophthalmopathies will be rewarding.
Based on various activities of TSHRAb from each patient, the patients could be classified into 7 subgroups according to the characteristics of TSHRAb they have. Group 1 (n=41) was characterized by moderate TSAb activities both in CHO-hTSHR cells and in FRTL-5 cells, typical TSAb epitope, rare blocking antibodies and high TBII activities. This group included the largest numbers of patients and seemed to be the typical TSHRAb of Graves’ disease. Group 2 (n=16) was characterized by the presence of blocking antibodies in most patients, albeit the other characteristics were the same as those in Group 1. Group 3 (n=19) patients had low TSAb activities both in CHO-hTSHR cells and in FRTL-5 cells, seldom had blocking antibodies, but they had high TBII activities. So, the patients in this group seemed to have a high titer of nonfunctional TSHRAb in their sera. Group 4 (n=30) could be categorized as ‘mild disease’ group, as they had low activities in all the assays for TSHRAb and had low antimicrosomal antibody activities. Group 5 (n=14) was characterized by moderate TSAb activities with atypical epitope (s), rare blocking antibodies and moderate TBII activities. Group 6 (n=10) patients had very high TSAb activities with typical epitopes, seldom blocking antibodies and low TBII activities. Group 7 (n=6) was characterized by very high TSAb activities with atypical epitopes and high TBII activities. The presence of blocking antibody could not be determined because of very high TSAb activities for Mc2 cell in this group.
Pretreatment serum thyroid hormone level was low only in group 4 patients compared to the other 6 groups (p<0.05). It suggests that the differences in epitopes for TSAb or the presence of blocking TSHRAb is not a major factor in determining the degree of thyrotoxicosis in Graves’ disease. The size of the goiter was significantly larger in those in group 1 and group 3 (p<0.05) compared to the other 5 groups. Marked differences in TSAb activities (group 1 higher) between group 1 and group 3 imply that the promotion of growth and the stimulation of function may be exerted by different populations of TSHRAb in Graves’ disease.
The TBII activities of patients were correlated with the disease activities only in group 1 and in group 2 patients. Thus, only in 40% of patients with Graves’ disease do TBII activities reflect the disease activity. This confirms that TBII epitopes of Graves’ disease may differ from epitopes for functionally relevant stimulating TSHRAb in many patients, as previous studies using monoclonal TSHRAbs found.
The group 3 patients had relatively large goiter and similar degrees of thyrotoxicosis despite low stimulating TSHRAb activities both in CHO-hTSHR cells and in FRTL-5 cells. They had very high TBII activities despite the rarity of blocking TSHRAb. So, they seem to have non-functional TBII in high titer sera. Interestingly, the antimicrosomal antibody titer, not the TBII or stimulating TSHRAb activities, was significantly associated with goiter size and with the degree of thyrotoxicosis. Therefore, the cellular infiltration and follicular cell destruction might have significantly contributed to development of goiter and thyrotoxicosis, respectively, in this subset of the patients. Histologic examination of thyroid in those patients would be rewarding.
In conclusion, by the classification of Graves’ disease according to the characteristics of TSHRAb they have, we could get a better understanding of the clinical implications of the epitope heterogeneity of TSHRAb in those patients and found, for the first time, the association of blocking TSHRAb with the presence of ophthalmopathy.
Acknowledgments
This study was supported in part by a grant #97-N1-02-04-A-10 of BIOTECH 2000, Ministry of Science and Technology, #HMP-97-G-2-21 of HAN (highly advanced national) project, Ministry of Health and Welfare, ROK.
Figure. 1.
Correlation between TSAb activities measured by wild-type hTSHR-CHO cells and those measured by FRTL-5 cells in 136 patients with Graves’ disease. TSAb activities were measured with crude IgG fraction of same concentration in both assays. Each point represents the mean of duplicate measurements.
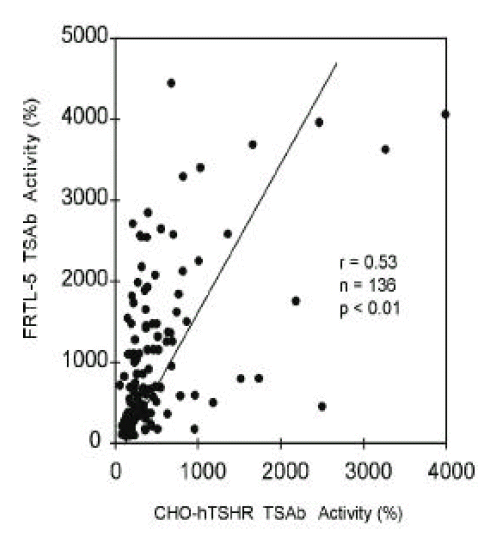
Figure 2.
Thyroid stimulating antibody activities measured by chimeric receptor expressing cells, Mc1+2 and Mc2, in 136 patients with Graves’ disease. Lines denote the positive cut-off values for each assay (174% in Mc1+2 cell and 153% in Mc2 cell assay). Each point represents the mean of duplicate measurements.
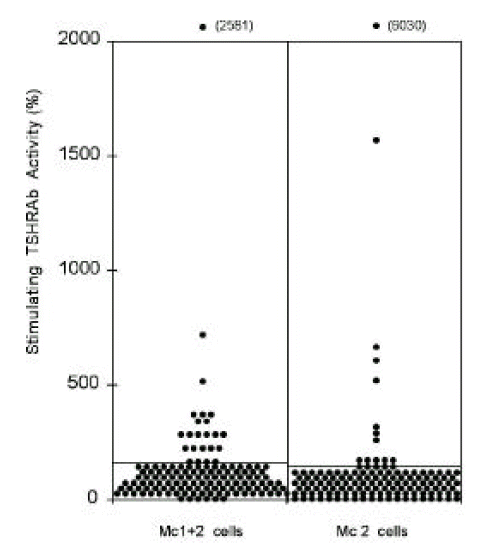
Figure 3.
Stimulation of cAMP production by bTSH or pooled Graves’ IgG in Mc2 cell line. Stimulation of cAMP by pooled Graves’ IgG (5 g/L) was negligible when compared to that by bTSH in Mc2 cell line expressing mutant TSH receptors, in which a.a. residue 90–165 of human TSH receptor was replaced by those of rat LH/CG receptors. Graves’ IgG were prepared from pooled sera of 30 untreated Graves’ patients.
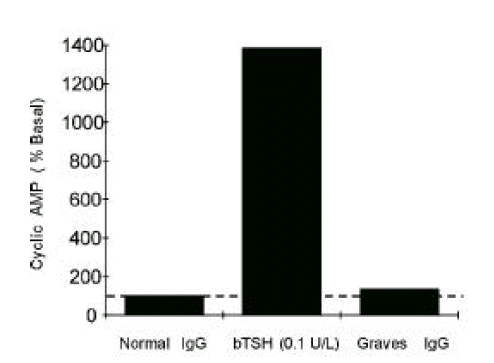
Figure 4.
TSBAb activities measured by chimeric receptor expressing cells, Mc2, in 136 patients with Graves’ disease. Line denotes the positive cut-off value (28.0%). Each point represents the mean of duplicate measurements.
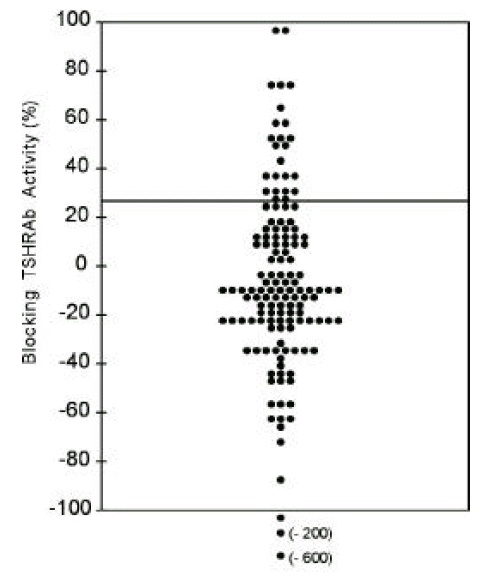
Figure 5.
Correlation between TBII activities and clinical parameters of patients in group 1. Initial serum T3 levels or degrees of goiter were significantly correlated with TBII activities.
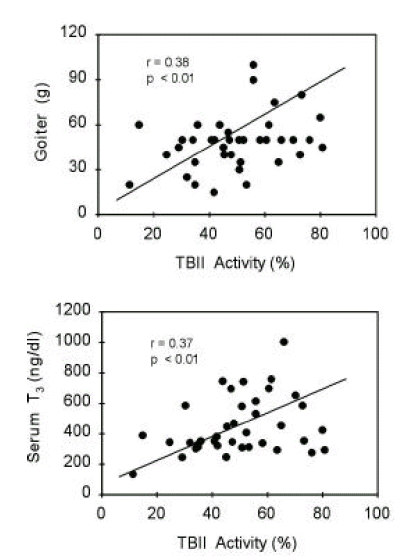
Figure 6.
Correlation between TBII activities and clinical parameters of patients in group 2. Initial serum T3 levels or degrees of goiter were significantly correlated with TBII activities.
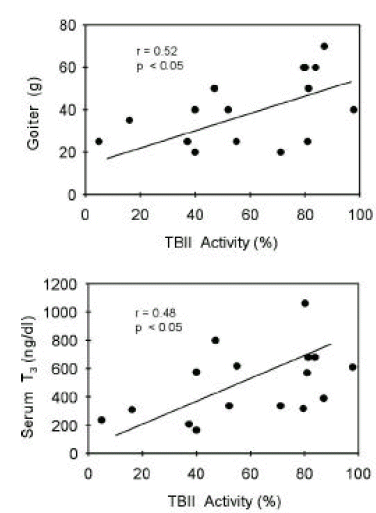
Figure 7.
Correlation between antimicrosomal antibody activities and clinical parameters of patients in group 3. Initial serum free T4 levels or degrees of goiter were significantly correlated with AMA activities.
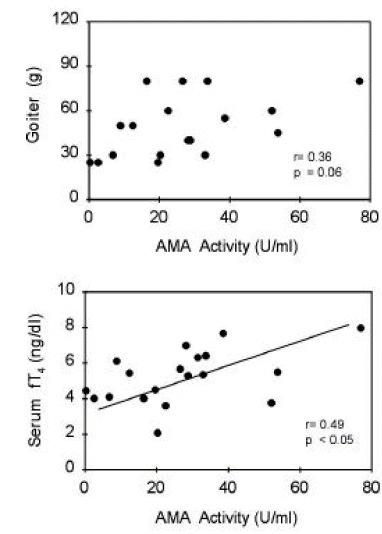
Table 1.
Clinical characteristics and initial laboratory data of 136 patients with Graves’ disease enrolled in study
Table 2.
Comparison of characteristics of TSH receptor antibodies between patients with blocking TSHRAb and those without blocking TSHRAb in Graves’ disease
| TSBAb-positive (n=24) | TSBAb-negative (n=112) | |
|---|---|---|
| CHO TSAb activity (%) | 309 ± 182 | 518 ± 621* |
| FRTL-5 TSAb activity (%) | 1131 ± 1107 | 1022 ± 939 |
| Mc1+2 TSAb | ||
| positive cases | 6/24 | 17/112 |
| mean activity (%) | 214 ± 509 | 121 ± 103 |
| Mc2 TSAb | ||
| positive cases | 2/24 | 16/112 |
| mean activity (%) | 116 ± 105 | 188 ± 581* |
| TBII activity (%) | 52.3 ± 26.5 | 43.0 ± 22.5† |
Table 3.
Comparison of clinical characteristics between patients with blocking TSHRAb and those without blocking TSHRAb in Graves’ disease
| TSBAb-positive (n=24) | TSBAb-negative (n=112) | |
|---|---|---|
| Age (years) | 38 ± 15 | 38 ± 13 |
| Sex (M:F) | 6:18 | 30:82 |
| Debut/Recurred (cases) | 20/4 | 94/18 |
| Family history of thyroid ds. (present) | 7/24 | 30/112 |
| Ophthalmopathy (present) | 11/24 | 30/112* |
| Goiter (grams) | 38 ± 15 | 45 ± 20 |
| Serum T3 (ng/dL) | 454 ± 238 | 393 ± 180 |
| Serum free T4 (ng/dL) | 4.41 ± 1.80 | 4.99 ± 2.55 |
| Antimicrosomal antibody | ||
| positive cases | 21/24 | 98/112 |
| activity (U/mL) | 20.0 ± 15.8 | 23.1 ± 19.7 |
| Antithyroglobulin antibody | ||
| positive cases | 11/24 | 72/112 |
Table 4.
Result of multiple linear logistic regression analysis of various TSHRAb activities predicting the presence of ophthalmopathy in patients with Graves’ disease
Table 5.
Result of cluster analysis based on various activities of TSH receptor antibodies in patients with Graves’ disease
Table 6.
Classification of patients according to the characteristics of TSH receptor antibodies in patients with Graves’ disease
Table 7.
Clinical characteristics of patients in subgroups classified by the characteristics of TSH receptor antibodies in patients with Graves’ disease
Table 8.
Correlation coefficients between TBII activities and clinical parameters in sub- groups classified by the characteristics of TSHRAbs in patients with Graves’ disease
Categorization of patients into the 7 groups was the same as that in Table 5.
REFERENCES
1. Kohn LD, Kosugi S, Ban T, Saji M, Ikuyama S, Giuliani C, Hidaka A, Shimura H, Akamizu T, Tahara K, Moriarty J, Prabhakar BS, Singer DS. 1992;Molecular basis for the autoreactivity against thyroid stimulating hormone receptor. Int Rev Immunol 9:135–165.


2. Tahara K, Yamamoto K, Yoshida S. 1992;Demonstration of two types of thyroid stimulating antibodies with different epitopes on the human thyrotropin receptor. Thyroid 2(Suppl):113.


3. Nagayama Y, Rapoport B. 1992;Thyroid stimulatory autoantibodies in different patients with autoimmune thyroid disease do not all recognize the same components of the human thyrotropin receptor: Selective role of receptor amino acids Ser25-Glu30. J Clin Endocrinol Metab 75:1425–1430.



4. Endo T, Ohmori M, Ikeda M, Anzai E, Onaya T. 1992;Heterogenous response of recombinant human thyrotropin receptor to immunoglobulins from patients with Graves’ disease. Biochem Biophys Res Commun 186:1391–1396.


5. Ueda Y, Sugawa H, Akamizu T, Okuda J, Ueda M, Kosugi S, Ohta C, Kihou Y, Mori T. 1995;Thyroid-stimulating antibodies in sera from patients with Graves’ disease are heterogenous in epitope recognition. Eur J Endocrinol 132:62–68.


6. Kim WB, Cho BY, Park HY, Lee HK, Kohn LD, Tahara K, Koh C-S. 1996;Epitopes for thyroid-stimulating antibodies in Graves’ sera: A possible link of heterogeneity to differences in responses to antithyroid drug treatment. J Clin Endocrinol Metab 81:1758–1767.


7. Watanabe Y, Tahara K, Hirai A, Tada H, Kohn LD, Amino N. 1997;Subtypes of anti-TSH receptor antibodies classified by various assays using CHO cells expressing wild-type or chimeric human TSH receptor. Thyroid 7:13–19.


8. Valente WA, Vitti P, Rotella CM, et al. 1983;Antibodies that promote thyroid growth: a distinct population of thyroid-stimulating autoantibodies. N Engl J Med 309:1028–1033.


9. Di Cerbo A, Di Paola R, Bonati M, Zingrillo M, De Filippis V, Corda D. 1995;Subgroups of Graves’ patients identified on the basis of the biochemical activities of their immunoglobulins.

10. Valente WA, Vitti P, Yavin Z, Yavin E, Rotella CM, Grollman EF, Toccafondi RS, Kohn LD. 1982;Monoclonal antibodies to the thyrotropin receptor: Stimulating and blocking antibodies derived from the lymphocytes of patients with Graves’ disease. Proc Natl Acad Sci USA 79:6680–6684.



11. Morgenthaler NG, Kim MR, Tremble J, Huang GC, Richter W, Gupta M, Scherbaum WA, McGregor AM, Banga JP. 1996;Human immunoglobulin G autoantibodies to the thyrotropin receptor from Ebstein-Barr virus-transformed B lymphocytes: characterization by immunoprecipitation with recombinant antigen and biological activity. J Clin Endocrinol Metab 81:3155–3161.


12. Kim WB, Chung HK, Lee HK, Kohn LD, Tahara K, Cho BY. 1997;Changes in epitopes for thyroid-stimulating antibodies in Graves’ disease sera during treatment of hyperthyroidism: Therapeutic implications. J Clin Endocrinol Metab 82:1953–1959.


13. Tahara K, Ban T, Minegishi T, Kohn LD. 1991;Immunoglobulins from Graves’ disease patients interact with different sites on TSH receptor / LH-CG receptor chimeras than either TSH or immunoglobulins from idiopathic myxedema patients. Biochem Biophys Res Commun 179:70–77.


14. Hidaka A, Ban T, Panesar NS, Minegishi T, Kohn LD, Tahara K. 1994;The extracellular domain of the lutropin / chorionic gonadotropin receptor has distinct sites important for agonist activity and high affinity binding: the agonist site is fully responsive to thyrotropin. Thyroid 4:447–457.


15. Kohn LD, Valente WA, Grollman EF, Aloj SM, Vitti P. 1986;Clinical determination and / or quantification of thytropin and a variety of thyroid stimulatory or inhibitory factors performed in vitro with an improved thyroid cell line FRTL-5. US Patent 4(609):622.

16. Southgate K, Creagh F, Teece M, Kingwood C, Rees Smith B. 1984;A receptor assay of the measurement of TSH receptor antibodies in unextracted serum. Clin Endocrinol (Oxf) 20:539–548.


17. Patibandla SA, Seetharamaiah GS, Dallas JS, et al. 1997;Different reactivities of recombinant glycosylated ectodomains of mouse and human thyrotropin receptors with patient autoantibodies. Endocrinology 138:1559–1566.


18. Akamizu T, Okuda J, Sugawa H, et al. 1995;Isolation of Ebstein-Barr virus-transformed lymphocytes producing IgG class monoclonal antibodies using a magnetic cell spreador (MACS): preparation of thyroid stimulating IgG antibodies from patients with Graves’ disease. Biochem Biophys Res Commun 207:985–993.


19. Watanabe Y, Tahara K, Hirai A, et al. 1997;Subtypes of anti-TSH receptor antibodies classified by various assays using CHO cells expressing wild-type or chimeric TSH receptor. Thyroid 7:13–19.


20. Ree Smith B, McLachlan SM, Furmaniak J. 1988;Autoantibodies to the thyrotropin receptor. Endocrine Reviews 9:106–121.


21. Kriss JP, Pleshakov V, Rosenblum AL, Holderness M, Sharp G, Utiger R. 1967;Studies on the pathogenesis of the ophthalmopathy of Graves’ disease. J Clin Endocrinol Metab 27:582–593.


22. Bonnyns M, Demeester-Mirkine N, Calay R, Bastenie PA. 1968;Evaluation of the relationship between long-acting thyroid stimulator, clinical and biological thyrotoxicosis and exophthalmos. Acta Endocrinol (Copenh) 58:581–592.


23. Pinchera A, Pinchera MG, Stanbury JB. 1965;Thyrotropin and long-acting thyroid stimulator in thyroid disease. J Clin Endocrinol Metab 25:189–208.


24. Lipman LM, Green DE, Snyder NJ, Nelson JC, Solomon DH. 1967;Relationship of long-acting thyroid stimulator to the clinical features and course of Graves’ disease. Am J Med 43:486–498.


25. Adams DD, Kennedy TH, Stewart RDH. 1974;Correlation between long-acting thyroid stimulator protector level and thyroid I–131 uptake in thyrotoxicosis. Br Med J 2:199–201.



26. Bech K. 1989;Thyroid antibodies in endocrine ophthalmopathy. A review. Acta Endocrinol (Copenh) 121(Suppl 2):117–122.
27. Wiersinga WM, Smit T, Van der Gaag R, Koornneef L. 1988;Temporal relationship between onset of Graves’ ophthalmopathy and onset of thyroidal Graves’ disease. J Endocrinol Invest 11:615–619.


28. Jacobson DH, Gorman CA. 1984;Endocrine ophthalmopathy: current ideas concerning etiology, pathogenesis and treatment. Endocrine Reviews 5:200–220.


29. Michelangelli VP, Poon C, Topliss DJ, Colman PG. 1995;Specific effects of radioiodine treatment on TSAb and TBAb levels in patients with Graves’ disease. Thyroid 5:171–176.


30. Tallstedt L, Lundell G, Torring O, et al. 1992;Occurrence of ophthalmopathy after treatment for Graves’ hyperthyroidism. N Engl J Med 326:1733–1738.


31. Karlson FA, Dahlberg PA, Jansson R, Westermark K, Enoksson P. 1989;Importance of TSH receptor activation in the development of severe endocrine ophthalmopathy. Acta Endocrinol (Copenh) 121(Suppl 2):132–141.
32. Kung AWC, Yau CC, Cheng A. 1994;The incidence of ophthalmopathy after radioiodine therapy for Graves’ disease: prognostic factors and the role of methimazole. J Clin Endocrinol Metab 79:542–546.


33. Murakami M, Miyashita K, Mizuma H, Yamada M, Iriuchijima T, Takeuchi T, Mori M. 1996;Discrete characteristics of antibodies raised against thyrotropin receptor-related peptides whose sequences are not conserved in the luteinizing hormone/chorionic gonadotropin receptor. J Clin Endocrinol Metab 81:1747–1752.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



