INTRODUCTION
Renal osteodystrophy is one of the most common complications in patients with end stage renal disease. Bone disorders are categorized into two principal types: a high-turnover osteodystrophy, known as osteitis fibrosa cystica and a low-turnover state characterized by adynamic bone disease1). At first, aluminum deposition was the principal factor thought to evoke the pathologic change of low-turnover osteodystrophy. But recently, low-turnover osteodystrophy occurs in many patients who have no evidence of excess aluminum accumulation and “relative hypoparathyroidism” is thought to play a key role in its pathogenesis2–7). Factors such as calcium, vitamin D and phosphate are known to be involved in regulation of secretion and synthesis of parathyroid hormone (PTH). In addition to these factors, it was reported that magnesium plays an important role in regulation of parathyroid hormone level13, 14).
Patients with chronic renal failure have an increased body magnesium content. In subjects on hemodialysis and peritoneal dialysis, the serum magnesium concentration parallels the dialysate magnesium level. A reduction of magnesium concentration in the dialysate produces a decrease in the serum Mg concentration and vice versa15–18).
Navarro et al demonstrated that there was a significant inverse correlation between serum iPTH level and serum magnesium level in a study using peritoneal dialysate containing 1.5 mEq/L of magnesium14). However, low-magnesium (0.5 mEq/L or 0.75 mEq/L) peritoneal dialysate, rather than high-magnesium, is used widely in Korea. In this paper, we evaluated the relationship between the serum parathyroid hormone and the magnesium levels in CAPD patients using low-magnesium peritoneal dialysate.
MATERIALS AND METHODS
The authors studied 56 patients who had been on CAPD for more than 6 months without any significant problems and who had been followed by Chonnam National University Hospital. The dialysate was composed of 132 mEq/L of Na, 96 mEq/L of CI, 3.5 mEq/L of Ca, 0.5 mEq/L of Mg and 40 mEq/L of lactate. All patients were dialyzed with 2 L of dialysate 4 times a day. Only those who had never taken calcitonin or aluminum hydroxide as a phosphate binder were included in this study.
Biochemical parameters, such as BUN, creatinine, alkaline phosphatase bony isoenzyme, total proein, albumin, total calcium, ionized calcium and intact parathyroid hormone level were measured. History taking was done at the point of blood sampling.
For the statistical analysis, computations were performed with SPSS 10.0 package for Windows and all the measurements were presented as mean±standard deviation. Student’s t-test was used to compare the mean of quantitative variables between groups. p less than 0.05 is considered significant. To measure the correlation between the serum parathyroid hormone level and other parameters, Pearson’s correlation analysis was used.
RESULTS
1. Demographic characteristics and laborarory parameters of the 56 patients included in the study
Table 1 lists demographic and biochemical parameters of the 56 patients included in the study. Among the 56 patients involved in this study, 34 were male and 22 were female. The mean age was 46.4±12.9 (21–68) years and the mean duration on peritoneal dialysis was 32.3±27.9 (6–132) months. The causes of the end stage renal disease were diabetes mellitus in 8 patients (14.3%), chronic glomerulonephritis in 5 patients (8.9%), and hypertension in 4 patients (8.9%). The remaining 38 patients (67.9%) were of unknown etiology. The mean serum magnesium level was 1.99±0.36 mEq/L. Among the total 56 patients, 15 patients (26.8%) showed hypermagnesemia (serum magnesium level >2.2 mEq/L) and 5 patients (8.9%) showed hypomagnesemia (serum magnesium level <1.6 mEq/L, Table 1).
2. Relationship between serum iPTH levels and serum magnesium, total and ionized serum calcium, alkaline phosphatase bony isoenzyme, inorganic phosphate levels among all 56 CAPD patients
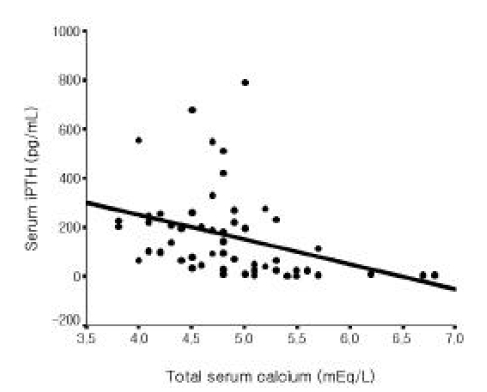
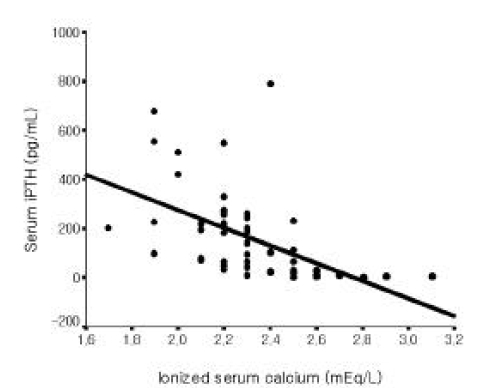
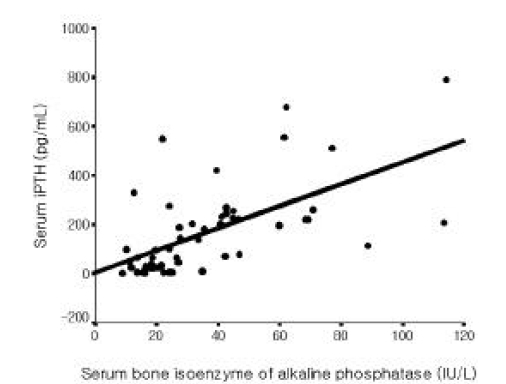
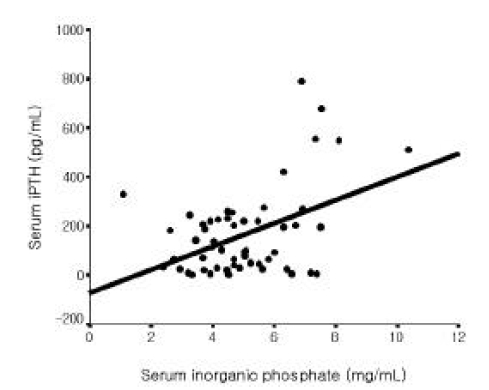
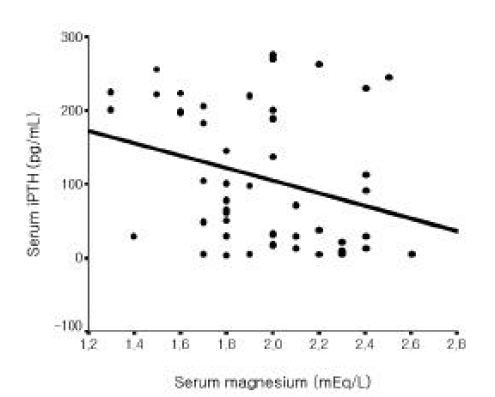
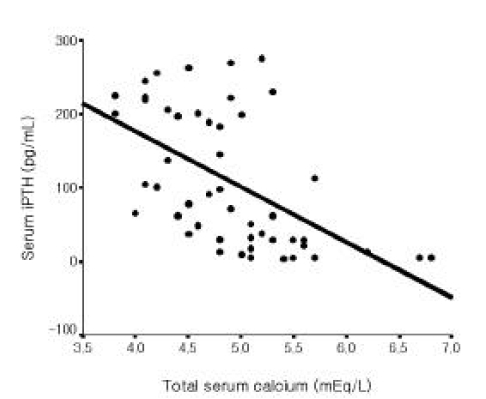
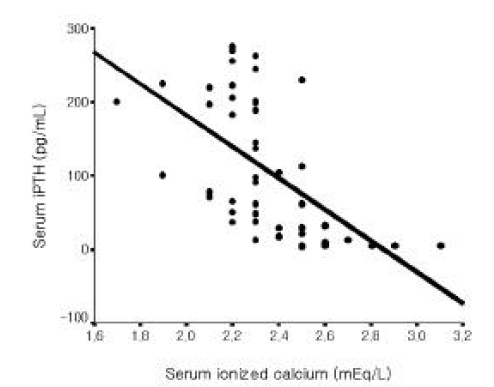
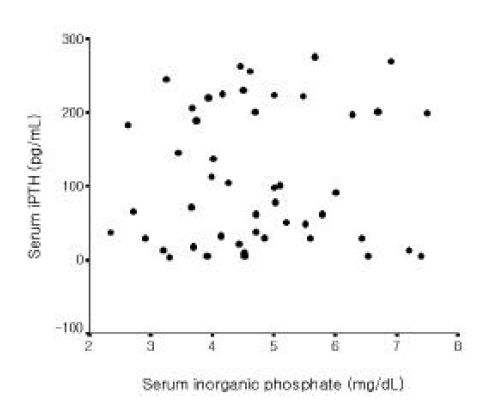
Among all 56 patients, serum iPTH level was not correlated with serum magnesium level (Figure 1). But it was inversely correlated with serum total calcium and ionized calcium levels, respectively (r=−0.365, p =0.006; r=−0.515 p<0.001, Figure 2, 3). Serum iPTH level was positively correlated with alkaline phosphatase bony isoenzyme level (r=0.612 p<0.001, Figure 4) and serum inorganic phosphate level (r=0.441 p=0.001, Figure 5).
3. Relationship between serum iPTH levels and serum magnesium, total and ionized serum calcium, alkaline phosphatase bony isoenzyme, inorganic phosphate levels among 49 CAPD patients whose serum iPTH levels are less than 300 pg/mL
Among 49 patients whose serum iPTH level was less than 300 pg/mL, serum iPTH level was inversely correlated with serum magnesium level (r=−0.295, p=0.039, Figure 6) and inversely correlated with serum total calcium and ionized calcium levels, respectively (r=−0.546, p<0.001; r=−0.572 p<0.001, Figure 7, 8). Serum iPTH level was positively correlated with alkaline phosphatase bony isoenzyme level (r=0.615, p<0.001, Figure 9). But there was no correlation between serum iPTH level and serum inorganic phosphate level (Figure 10).
4. Comparison between two groups classified according to serum iPTH level among 49 CAPD patients whose serum iPTH levels are less than 300 pg/mL
Among 49 patients whose serum iPTH level was less than 300 pg/mL, lower iPTH group (serum iPTH<120 pg/mL) showed higher serum magnesium level (p=0.037), higher serum total calcium level (p<0.001) and lower bone isoenzyme of alkaline phosphatase level (p<0.001) than those of higher iPTH group (120 pg/mL≤serum iPTH<300 pg/mL, Table 2).
DISCUSSION
It is well known that serum PTH level is regulated by calcium, phosphate and 1,25-dihydroxy vitamin D (1,25(OH)2D)8–21). Calcium exerts a negative feedback control on serum PTH level. A decrease in extracellular calcium leads not only to an increase in PTH secretion but also to increases in the mRNA expression of PTH and proliferation of parathyroid cell and release of PTH is suppressed during acute hypercalcemia8–11). Phosphate exerts indirect stimulatory effects on PTH secretion by decreasing renal production of calcitriol and by lowering plasma ionized calcium. It also plays a direct stimulatory role at the level of parathyroid gland9, 10, 12). Calcitriol diminishes transcription of PTH mRNA by binding to Vitamin D receptor and stimulates intestinal absorption of calcium and the skeletal mobilization of calcium, thereby increasing plasma calcium and inhibiting PTH secretion9–11).
Recently, in addition to these factors, magnesium is suggested to play an important role in regulating PTH level. An intravenous magnesium sulfate infusion significantly suppressed iPTH secretion in patients with primary hyperparathyroidism19). In addition, an intravenous magnesium sulfate infusion depressed iPTH levels in healthy men20, 21). The use of magnesium carbonate as a phosphate binder depressed serum PTH level22). Normalization of serum magnesium by using low dialysate magnesium in hypermagnesemic hemodialysis patients was followed by a rise in circulating PTH levels18, 23). There are reports that serum iPTH levels have a highly significant inverse correlation with serum magnesium level in hemodialysis and peritoneal dialysis patients13, 14) and in the author’s study which involved hemodialysis patients, there was a significant inverse correlation between serum iPTH levels and serum magnesium levels24). Magnesium is the fourth most abundant cation in the body. Of this, about 60% is in bone, 20% in skeletal muscle, 19% in other cells and only about 1% is found in the extracellular fluid17). Renal excretion is the major route of magnesium elimination from the body in health. As renal failure progresses, the fraction of filtered magnesium increases, but total filtration amount decreases. So, at last, absorption from the gastrointestinal tract exceeds excretion from the kidney16). In this context, hypermagnesemia is common in patients with advanced renal failure15–17). Dialysate magnesium plays a critical role in determining serum magnesium level in peritoneal dialysis patients15–18). Ashan et al observed that 21 of total 33 CAPD patients (64%) developed hypomagnesemia (serum magnesium level <1.25 mEq/L) when dialyzed with a low-magnesium (1.0 mEq/L) peritoneal dialysis solution25). Navarro et al reported that in a study involving 51 CAPD patients, 30 patients (59%) developed hypermagnesemia (serum magnesium level >2.02 mEq/L) when using 1.5 mEq/L-magnesium peritoneal dialysis solution and the mean serum magnesium level was 2.16±0.38 mEq/L14).
In the present study, the mean peritoneal dialysate calcium level was 3.5 mEq/L, the same as in Navarro’s study and the mean peritoneal dialysate magnesium level was 0.5 mEq/L lower than that in Navarro’s study. The mean serum magnesium level was 1.99±0.36 mEq/L, 15 patients (26.8%) showed hypermagnesemia (serum magnesium level >2.2 mEq/L) and 5 patients (8.9%) showed hypomagnesemia (serum magnesium level <1.6 mEq/L). Compared with Navarro’s study which used 1.5 mEq/L magnesium peritoneal dialysate (mean serum magnesium level: 2.16±0.38 mEq/L), this study shows that the mean serum magnesium concentration is lower and the incidence of hypermagnesemia is lower than that in Navarro’s study and 5 patients developed hypomagnesemia, as well. These results suggest that dialysate magnesium plays a critical role in determining serum magnesium level in peritoneal dialysis patients15–18). It is well known that serum iPTH level is correlated wtih bone forming rate, so serum iPTH level is regarded as a useful marker for detecting renal osteodystrophy3–7). Recently, it is reported that the incidence of low-turnover osteodystrophy, including adynamic bone disease, has increased5–7). Typically, these patients have relatively low levels of IPTH3–7). Fournier et al recommended that iPTH levels shoud be permitted to fluctuate between 100 and 300 pg/mL in patients on CAPD in order to have a normal bone forming rate26). Torres et al showed that an iPTH level below 120 pg/mL was highly predictive of low bone turnover in patients not treated with vitamin D7).
In the present study, serum iPTH level was not correlated with serum magnesium level among all 56 patients. However, it was inversely correlated with serum total calcium and ionized calcium levels. Serum iPTH level was positively correlated with alkaline phosphatase bony isoenzyme level and serum inorganic phosphate level. Seven patients whose iPTH levels were higher than 300 pg/mL were thought to be in high bone turnover state. Among only 49 patients whose serum iPTH level was less than 300 pg/mL, serum iPTH level was inversely correlated with serum magnesium level, and inversely correlated with serum total calcium and ionized calcium levels, respectively. Serum iPTH level was positively correlated with alkaline phosphatase bony isoenzyme level. But there was no correlation between serum iPTH level and serum inorganic phosphate level.
Brown et al discovered extracellular Ca2+-sensing receptor from bovine parathyroid27). This receptor is found in high concentrations on the surface of parathyroid cells, on C-cells of the thyroid, at various sites along the nephron, in brain, on bone cells and in other tissues. The Ca2+-sensing receptor is G-protein coupled receptor that on activation stimulates phospholipase C, which results in increased levels of inositol 1,3,5-triphosphate, which in turn elevates the cytosolic Ca2+ activity by mobilizing Ca from various intracellular sites. Activation of the Ca2+-sensing receptor reduces the secretion of PTH from the thyroid and increases urinary excretion of calcium from the kidney9, 28). The Ca2+-sensing receptor lacks specificity. Thus, it is stimulated by other trivalent elements such as gadolinium and by polycationic compounds, such as neomycin. The lack of specificity of Ca2+-sensing receptor explains how magnesium reduces the secretion of PTH21, 28).
In the present study, among all 56 patients there was no correlation between serum iPTH level and serum magnesium level but among only 49 patients whose serum iPTH level was less than 300 pg/mL, serum iPTH level was inversely correlated with serum magnesium level. The correlation between serum iPTH level and serum magnesium level was weaker than that of serum iPTH level and serum calcium level. These results suggest that serum calcium is more potent than serum magnesium in regulating serum iPTH level and are in line with other studies which compared the potency of magnesium with that of calcium in regulating serum iPTH level20, 29). The result shows weaker correlation between serum magnesium level and serum iPTH level than that of Navarro’s study which included CAPD patients14), and than that of the authors’ study which included hemodialysis patients24). In the above studies, the mean serum magnesium levels were higher than that of the present study, respectively. The difference of serum magnesium level is presumed to be due to the difference of peritoneal dialysate magnesium concentration or of the amount of magnesium intake. The peritoneal dialysate calcium concentration was 3.5 mEq/L, the same as in this study. But the peritoneal dialysate magnesium concentration of two forementioned studyies were 1.5 mEq/L and 1 mEq/L, respectively, higher than 0.5 mEq/L in this study. So, we suppose that the lower level of peritoneal dialysate magnesium led to lower serum magnesium level and as a result, to weaker iPTH suppressing effect of magnesium and weaker correlation between serum magnesium and iPTH level than the above-mentioned studies. The mean iPTH level (113±88 pg/mL) in the study by Navarro et al was lower than that (164±177 pg/mL) of the present study. The present study used lower level of peritoneal dialysate magnesium, and showed lower level of serum magnesium and higher level of serum iPTH than the study by Navarro et al. This supports the fact that peritoneal dialysate magnesium level affects the serum magnesium level and there is an inverse correlation between serum magnesium and serum iPTH level. It is noteworthy that when seven patients whose iPTH levels were higher than 300 pg/mL were included, the correlation between serum magnesium and serum iPTH level disappeared. These findings also suggest that, in patients with secondary hyperparathyroidism whose iPTH level is high, iPTH level may respond to negative feedback control by calcium whose potency is high, but not by magnesium whose potency is relatively lower than calcium.
In summary, among the CAPD patients whose serum iPTH level was less than 300 pg/mL, there was a significant inverse correlation between serum iPTH level and serum magnesium level. This study indicates that not only serum calcium level but also serum magnesium level are important in the regulation of serum iPTH levels of CAPD patients who have been dialyzed by low-magnesium peritoneal dialysate.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





