Acute Acalculous Cholecystitis Associated with Cholecystoduodenal Fistula and Duodenal Bleeding. A Case Report
Article information
Abstract
Although acute acalculous cholecystitis (AAC) accounts for less than 10% of acute cholecystitis in the adult population, gangrene and perforation are much more frequent compared to the usual cases of acute cholecystitis (calculus cholecystitis). However, spontaneous biliary-enteric fistula is well recognized in AAC, 90% of which are cholecystoduodenal fistula (CDF) though it is an uncommon disorder. The majority of the CDF are caused by cholelithiasis. As patients are usually associated with complicated clinical illness, the diagnosis is often difficult to make and required surgery is often delayed. We have studied a rare complication of acute acalculous cholecystitis which was presented as intermittent upper gastrointestinal bleeding. Ulceration of the superficial branch of the cystic artery has been observed due to acalculous cholecystitis associated with a cholecystoduodenal fistula. We have performed a transfixing ligation of the bleeding vessel, cholecystectomy and simple closure of the CDF. We have finally made a diagnosis of early gallbladder cancer through a frozen section. There was no serious complication after the operation and the patient has achieved an uneventful recovery.
INTRODUCTION
Most acute cholecystitis are caused by cholelithiasis but about 10% are acute acalculous cholecystitis1, 2). Causes of acalculous cholecystitis are severe trauma or burn, surgery, long-term starvation, cytomegalovirus, cryptosporidiosis, systemic infection such as Typhoid and severe underlying diseases (Diabetes Mellitus, Cardiovascular disease). The clinical course in such cases is so poor that mortality rate reaches about 10–50%1, 2). Perforation of the gallbladder commonly occurs in acalculous cholecystitis as one of the complications, a type of which is biliary-enteric fistula. About 10 cases of Bouveret's syndrome18), in which upper GI bleeding due to secondary invasion of the cystic artery by gallstones, which had obstructed the bulb of the duodenum have been reported, but there was no case of acalculous cholecystitis which is associated with cholecystoduodenal fistula and cystic arterial bleeding. Therefore, taking into consideration other literatures, we intend to report a case of cystic arterial bleeding due to acute acalculous cholecystitis, which was presented as upper GI bleeding via cholecystoduodenal fistula and which has undergone open cholecystectomy.
CASE REPORT
A 60-year-old man was admitted to hospital because of upper abdominal discomfort. The patient with a history of heavy alcohol intake, was admitted for upper abdominal discomfort and weight loss, the onset of which was about a month ago. Before admission, he had been to another hospital and the findings of abdominal computed tomography taken there had shown swelling of the gallbladder and mildly-dilated intrahepatic bile ducts. The patient had no past medical history of chronic liver disease.
On physical examination, the patient's blood pressure was 100/70 mmHg, pulse rate 84/min and body temperature 36.5°C. The patient was conscious and not acutely ill looking. Neither anemic conjunctivae nor icteric sclerae were shown. No abnormal findings on the head, ear, nose or throat were present. On auscultation, a regular heart sound without murmur was heard. On abdominal examination, there was right upper quadrant tenderness but no rebound tenderness. The laboratory study showed WBC 4,000/mm3 (4,000–10,000), Hb 12.7 g/dL (12–16), PLT 150,000/mm3 (130,000–400,000), AST 226 IU/L (∼40), ALT 219 IU/L (∼40), Total Bilirubin 1.1 mg/dL (0.1–1.2), ALP 588 IU/L (70–190), r-GT 391 IU/L (4–24), Amylase 76 U/L (30–110), Lipase 78 U/L (23–300), CA 19–9 32.62 U/mL (∼37), CEA 2.6 ng/mL (∼5). No specific findings were present in the simple abdomen radiographs. On the 2nd day of admission, abdominal ultrasonography revealed echogenic sludge materials within the distended gallbladder with no gallstone (Figure 1). On the 3rd day of admission, abdominal CT scan showed diffuse thickening of the gallbladder wall, high attenuated materials with a diameter of 2 cm and focal inflammatory stricture at the level of the common hepatic duct (Figure 2). On the 4th day of admission, endoscopic retrograde cholangiopancreatography revealed a mild narrowing of the distal common hepatic duct and diffusely dilated left intrahepatic duct. The gallbladder was not seen and no evidence of choledocholithiasis was present (Figure 3).
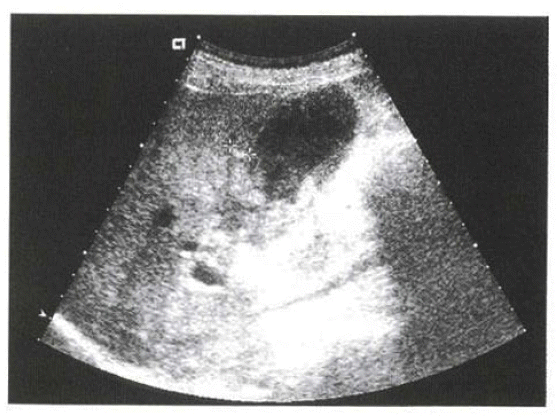
Abdominal ultrasonography on the 1st admission shows distended gallbladder with internal debris and sludge materials.
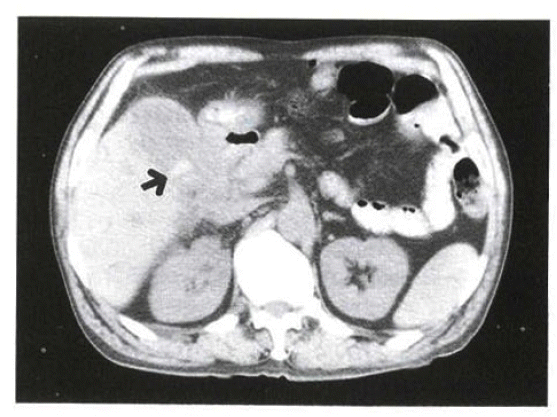
Abdominal CT on the 1st admission shows diffuse thickening of gallbladder wall and about 2cm sized highly-attenuated materials (arrow).

ERCP shows diffuse segmentally dilated Lt. IHD and slightly narrowed distal CHD (arrow). There is no filling defect in CBD and GB was invisible.
The treatment had begun with antibiotics under the presumptive diagnosis of acute acalculous cholecystitis and emergency esophagogastroduodenoscopy was done for melena and hematemesis that had occurred on the 7th day of admission. A round shallow ulcer with a diameter of 0.5 cm was noted at the junction of the 1st and 2nd duodenum and blood oozing at the ulcer base. The bleeding ceased after the injection of 1:1000 epinephrine (Figure 4). A follow-up endoscopic therapy was performed on the following day, due to about 200 mL of hematemesis.
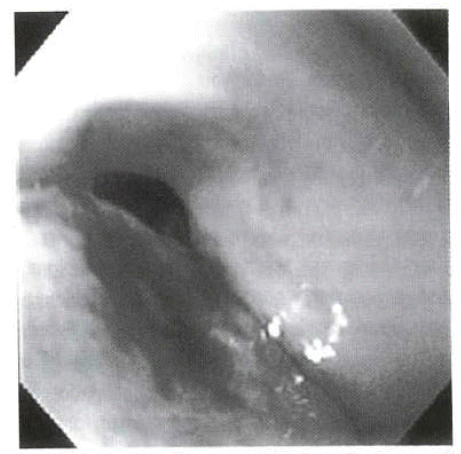
Endoscopy shows a round shallow ulcer base with blood oozing at the junction of the 1st and 2nd part of duodenum.
The patient was discharged with cholecystectomy on schedule and proton pump inhibitor (PPI) was prescribed for the treatment of duodenal ulcer. On the 16th day after the 1st occurrence of hematemesis he was readmitted for the recurrent hematemesis and melena. Immediately, emergency gastroduodenoscopy was done, but bleeding focus was not found. On the 5th day of the 2nd admission gastroduodenoscopy had showed only a small linear shallow ulceration at the duodenal bulb and that the bleeding had stopped. The abdominal ultrasonography taken on the 13th day of the 2nd admission has shown significantly thickened anterior wall of the gallbladder with echogenic mass (Figure 5). On the 14th day of the 2nd admission, open cholecystectomy was performed. The operative findings revealed thickening and edema of the gallbladder wall, adhesion of mesentery to the hepatoduodenal ligament and duodenum, and cystic arterial bleeding into the cholecystoduodenal fistula. The serosa was blotch and green-black discolored. No gross finding of gallbladder cancer was found around the neck of the gallbladder during the operation. Besides, as the operation was carried out under the diagnosis of acalculous cholecystitis, a frozen biopsy on the surrounding liver and the lymph nodes in the vicinity of the gallbladder was not performed. The gross findings have shown overall ulcerosuppurative inflammation on the fundus of gallbladder with necrosis and partially thickened anterior wall. The histologic finding was compatible with acute nonspecific suppurative inflammation, as most of the mucosae were detached with a partially high grade dysplastic change in the neck of gallbladder and focal superficial carcinomatous invasion into the muscle layers (Figure 6A, 6B, 6C). The patient had no post-operative complication and has been currently followed up without recurrence or complication at an outpatient clinic. This case is one in which a diagnosis of gallbladder cancer was made by histology after surgery.

Abdominal US shows eccentric wall-thickening of anterior wall of GB and there was echogenic mass filling the GB lumen. Air trapping in the anterior wall of GB and biliary tract is also seen.
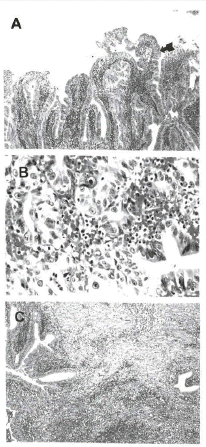
GB biopsy findings. (A) Microscopy shows diffuse epithelial hyperplasia with partially high-grade dysplasia on GB neck (arrow) (H&E, ×40). (B) Microscopy shows focal areas of carcinomatous change on GB neck (H&E, ×100). (C) Microscopy shows acute nonspecific suppurative inflammation on GB fundus. (H&E, ×40).
DISCUSSION
The most common cause of acute cholecystitis is the gallstone, but acalculous cholecystitis occurs in 5–15%, especially in elderly males. However, its incidence in patients who have undergone major surgery or children is as relatively high as 87% and 50% respectively1–3). The definite cause or pathophysiology is not in clear yet and, in most cases, multiple factors are thought to be associated. The possible causes are ones that cause increased viscosity and stasis of bile juice, such as narcotics and long-term starvation after surgery or trauma, sepsis, high fever, dehydration or massive transfusion. In this case, no specific pre-existing disease like Diabetes Mellitus was present. The serologic test for Clonorchiasis was positive, but there was no specific radiologic finding. The serologic tests for Typhoid fever came out negative. Diagnosis of the disease used to be made after trauma, surgery and at ICU setting, but now the rate of its diagnosis at outpatient clinics is on the increase. In most cases reported by Kim et al.4) and others in Korea, patients were referred to an emergency room or outpatient clinic with acute presentation and diagnosis of it.
The clinical manifestations are different from those of calculous cholecystitis. Right upper quadrant pain, fever and localized pain on the region of the gallbladder and leukocytosis might occur, but most are asymptomatic in patients, especially after surgery or the elderly. Fever of unknown origin or hyperamylasemia can be helpful in establishing a diagnosis. At the early stage, specific symptoms and other physical findings on the right upper quadrant can be absent in about 75% of patients. At the time of diagnosis, complications, such as gangrene or perforation of the gallbladder, can occur in about half of patients. Gangrene and perforation of the gallbladder are the most severe complications of acute cholecystitis occurring in 2–18% and bring about a much higher mortality rate (16–26%) than acute cholecystitis without perforation8, 10, 11). Perforation of the gallbladder as a complication of acute cholecystitis was first described by Duncan et al.5) in 1884. Acute perforation of the gallbladder was classified into 3 types by Niemeier6) in 1934 and then the 4th type was added by Anderson et al.7) in 1987. That is, Type I is acute perforation that causes peritonitis, Type II is subacute perforation that forms a local abscess around the gallbladder, Type III is chronic perforation that forms a fistula with the surrounding intestine and Type IV is one that forms cholecystobiliary fistula. Perforation of the gallbladder is likely to be thought as Type I acute perforation only, but all of the four types are included in cases of perforation of the gallbladder. In this case, the perforation given rise to was Type III with formation of a cholecysto-duodenal fistula.
Diagnostic tools available for perforation of the gallbladder and cholecystointestinal fistula are abdominal ultrasonography, computed tomography, hepatobiliary scan and endoscopic retrograde cholangiopancreatography (ERCP). ERCP can detect perforation of the gallbladder by directly observing the leakage of contrast media from the cavity of the gallbladder into the surrounding tissues and make a diagnosis of fistula into the adjacent organs. However, for ERCP to diagnose perforation of the gallbladder, the cystic duct should be patent and, also, there is a risk of ascending infection after the procedure. In the study done by Ham et al.9), the site of perforation confirmed by surgery was reported to be identical to the one diagnosed by ERCP. However, in this case, ERCP could not detect leakage of contrast media because of the obstructed cystic duct, therefore, the diagnosis of cholecysto- duodenal fistula had not been made until surgery was done. Incidence of cholecystointestinal fistula is reported to be 0.1–0.5% in autopsy and reaches 1.2–5% in cases of cholecystectomy1, 12).
Ninety percent of cholecystoduodenal fistula occurs in the gallbladder wall, especially where a stone causes erosion in the neck. Besides, about 10% of fistula occurs in the duodenal ulcer, suture sites and gallbladder cancer1, 13). The most common cause of cholecystoduodenal fistula is a gallstone, but that of choledochoduodenal fistula is a duodenal ulcer in 80%1). Cholecystointestinal fistula brings about a complication in 0.3–0.5% of patients with a gallstone. The common fistula, in order, are cholecystoduodenal fistula (60%), cholecystocolonic fistula (17%), cholecystogastric fistula (5%) and choledochoduodenal fistula (5%). Cholecystoduodenal fistula commonly accompanies diseases that require surgical treatment, so the cause of the fistula is an important factor in deciding an operative method and technique. The classic therapy is open-cholecystectomy with closure of the fistula. Recently, Oka et al.13) have reported operative cases of cholecystocolonic fistula and cholecystoduodenal fistula, and Carlei et al.14) have reported five successful cases of laparoscopic surgery on cholecystoduodenal fistula. As cholecystoduodenal fistula without a complication caused by a gallstone, follows its spontaneous closure and long-term follow-ups of patients have revealed no specific clinical problems, an endoscopic follow-up for cases without a complication of fistula is rather more necessary than open-cholecystectomy15). Therefore, for cases with no local or systemic risk factors and a favorable clinical course with medical therapy, conservative treatment is enough to expect recovery of a patient's condition.
Invasion of the cystic artery in a duodenal ulcer is very rare. Ring et al.16) in 1972 had first reported a case of invasion of the superficial branch of the cystic artery in a duodenal ulcer, and Iwamura et al.17) had reported a case of massive upper gastrointestinal bleeding caused by invasion of the duodenal ulcer into the cystic artery. Invasion of a cystic artery should be considered in a duodenal ulcer if massive bleeding occurs in the anterior wall or below the bulb. In addition, cases of bleeding from the cystic artery in cholecystoduodenal fistula caused by a gallstone had been reported in literatures. In Bouveret's syndrome, secondary bleeding from the duodenal ulcer caused by a gallstone is very rare. A gallstone that completely impacts the duodenum can cause erosion in the artery and consequently bleeding in the duodenum, which may be misdiagnosed as a duodenal ulcer. In Bouveret's syndrome, less than 10 cases associated with upper gastrointestinal bleeding had been reported. Invasion of the cystic artery and resultant bleeding by a gallstone that impacts the duodenal bulb completely had been reported by Heinrich et al.18). The case in this study is thought to be a very rare case of invasion of the cystic artery by spontaneous fistula due to acalculous cholecystitis, unlike cystic arterial bleeding by the duodenal ulcer or invasion and bleeding of cystic artery by a gallstone reported in above-mentioned literatures. In acute cholecystitis, the cystic duct is obstructed by edema or stones, which gradually leads to swelling and severe inflammation of the gallbladder. In consequence, blood flow to the gallbladder is cut off and ischemia, necrosis and, eventually, perforation of the gallbladder takes place. In patients with accompanying atherosclerotic vascular diseases or low cardiac output, ischemic necrosis and perforation of the gallbladder can occur and in occasionally due to the obstruction of the cystic duct by a tumor. The most common site for perforation of the gallbladder is the fundus and it coincides with its low blood supply. Therefore, it is thought that the cholecystoduodenal fistula was brought about as a complication of the inflammation and, with associated erosion of the cystic artery, bleeding occurred into the gallbladder. The hemorrhage was thought to be then drained out through the cholecystoduodenal fistula.
As primary cancers of the biliary tract are non-specifically similar to benign disease, an early diagnosis is considerably hard to make. Therefore, at the time of detection of those cancers, invasion of cancer cells into nearby major organs makes radical resection very difficult.
Hohaus et al.19) have reported that the incidence rate of gallbladder cancer incidentally found after surgery among benign hepatobiliary diseases is 1–2%. In contrast, in an autopsy study, about 0.5% shows gallbladder adenocarcinoma. It is presumed that the introduction of laparoscopic cholecystectomy along with the recent development of diagnostic imaging technology, such as ultrasonography and computed tomography, may have increased the detection rate of an early gallbladder cancer. Most medical practitioners agree that cholecystectomy should be performed for a primary cancer of the gallbladder which is localized to the mucosa or muscle layers of the gallbladder, and hepatic resection with radical lymph node dissection for an advaned cancer which has progressed beyond muscle layers. For a more aggressive treatment some attempt cholecystectomy for cancers localized to only the mucosa, extended cholecystectomy with partial hepatectomy for those invading beyond mucosa, pancreatoduodenectomy for those with metastasis to the posterior pancreatic lymph nodes, and extended hepatectomy for those invading the liver. Ogura et al.20) had reported that lymph node metastasis had been detected in 15% of cancers invading the muscle layers of the gallbladder (T1b), which also had been noted in 13% of cancers with stage T1b in the study done by Hwang et al.21) Therefore, due to its poor prognosis, more aggressive surgery is required for an inflammatory gallbladder disease with a complicated early gallbladder cancer. In consequence, lymph node dissection is necessary for a primary cancer invading the muscle layers and, on the contrary, no lymph node metastasis should be confirmed to expect prognosis of an early cancer to some extent. It had been reported that, for an advanced gallbladder cancer, an application of an extended complete resection based on the mechanism of progression had achieved an improvement in the survival rate. The patient in this case is expected to have further aggressive follow-ups as neither confirmation of no lymph node metastasis was made nor an extended cholecystectomy was performed. In this case the ultrasonography and computed tomography showed overall wall thickening of the gallbladder neck, but no radiologic features supporting gallbladder cancer were found. In surgical specimens obtained, high-grade dysplastic and carcinomatous changes invading the superficial muscular layers of the gallbladder neck were shown, but were irrelevant in location to the fundus of the gallbladder where the fistula was formed.
In summary, the major pathologic cause of cholecystointestinal fistula is a gallstone. In acute acalculous cholecystitis, cholecystoduodenal fistula as a complication is very rare. Therefore, taking consideration into many other literatures, we have reported a case of acute acalculous cholecystitis for which an early misdiagnosis of duodenal ulcer bleeding was made, but in which, finally, correction and confirmation of its diagnosis as acute acalculous cholecystitis was made by open-cholecystectmoy in the presence of cholecystoduodenal fistula, cystic arterial bleeding and, at the same time, accompanying early gallbladder cancer.