A Less Intensive Combination of Paclitaxel and Carboplatin in Advanced Non-small Cell Lung Cancer Patients who Have Aged 60 Years or More and Has a Poor Performance Status
Article information
Abstract
Background
The aim of this study was to evaluate the response, survival, and toxicities of a less intensive combination of paclitaxel and carboplatin, which is used in advanced non-small cell lung cancer (NSCLC) patients older than 60 years of age including those with a poor performance status.
Methods
Thirty patients received 135 mg/m2 of paclitaxel on day 1, and carboplatin was administered to the patients on day 1 every 4 weeks over an area under the concentration-time curve of 6.
Results
The response rate was 40%, the median overall survival was 9.1 months (95% CI, 4.2 to 14 months), and the 1 year survival rate was 31%. The median progression-free survival was 7.7 months (95% CI, 3.1 to 12.2 months). In addition, the toxicities were generally mild and reversible.
Conclusion
This study demonstrates that a less intensive combination of paclitaxel/carboplatin is active and well tolerated in advanced NSCLC patients who are older than 60 years including those with a poor PS 3–4.
INTRODUCTION
According to the reports of the Korea National Statistical Office, it has been estimated that 25 out of 100,000 deaths are due to lung cancer in Korea, which makes it the leading cause of cancer-related mortality in the year 2001. Aging is associated with an increased incidence of lung cancer, and the peak incidence occurs between the ages of 60–65. In Korea, approximately 65% of patients are 60 years of age or older1, 2). Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung tumors, and more than 80% of the NSCLC patients were found to be in the advanced stage III and stage IV1). In patients with stage III NSCLC, chemotherapy in combination with thoracic radiation is the standard procedure of care. Unfortunately, there are subsets of patients with stage III NSCLC in whom the combined therapeutic modalities are unsuitable, such as patients with a malignant pleural effusion or a poor performance status (PS). These patients are generally grouped with the stage IV patients when systemic chemotherapy is considered.
Since 1990, the standard chemotherapy for patients with an unresectable disease and a good PS (ECOG level 0 or 1) consisted of cisplatin or carboplatin combined with paclitaxel, gemcitabine, vinorelbine, and docetaxel3–5). Several trials have reported that the paclitaxel and carboplatin combination was well tolerated, and the combination was more convenient for these patients and was not significantly inferior to the other regimens5, 6).
However, there are questions that persist regarding the use of ‘standard’ regimens for patients who are elderly and have a poor PS, Therefore, this study was performed to evaluate the response, survival, and toxicities of a less intensive combination of paclitaxel and carboplatin in these advanced NSCLC patients.
MATERIALS AND METHODS
Between October 2001 and February 2003, thirty patients from Ulsan University Hospital were enrolled in this study.
1. Eligibility criteria
Chemotherapy-naïve patients≥60 years of age were eligible if they had a histologically or cytologically confirmed locally advanced (IIIB) or metastatic (IV) NSCLC. No prior chemotherapy or thoracic radiotherapy was permitted. The subjects were required to have adequate bone marrow function (absolute neutrophil count≥1,500/mm3, platelet count≥100,000/mm3), adequate hepatic function (bilirubin level two times the upper limit of the normal, AST and/or ALT<three times the upper limit of normal) and an adequate renal function (creatinine<1.5 mg/dL) Brain metastasis, if asymptomatic, was not considered as an exclusion criteria. Also, all the patients provided an informed consent.
2. Treatment Schedule
135 mg of paclitaxel per square meter of body-surface area was administered over a one hour infusion, which was followed by the administration of carboplatin on day 1 of a four-week cycle at a dose calculated to produce an area under the concentration-time curve of 6.0 mg per milliliter per minute. The doses of paclitaxel and carboplatin were reduced by 25% for all other grade 3 toxicities (except alopecia) and were omitted entirely for any grade 4 toxicities.
3. Pretreatment and follow-up studies
Prior to participating in the study, all patients were required to have a complete physical examination, chest X-ray, chest computed tomographic scan, complete blood counts (CBC), biochemical tests, and an ECG. The physical examination, chest X-rays, CBC, biochemical tests were performed on each patient before each cycle and 14 days after each cycle of therapy.
4. Response and Toxicity Evaluation
The patients who received at least two treatment cycles were assessed for their response unless they showed definitive evidence of progression after the first cycle. Patients who had received at least one treatment cycle were assessed for toxicity. The responses were graded according to the RECIST criteria7). Complete remission (CR) was defined as the disappearance of all the known lesions, no emergence of new lesions, and normalization of the tumor markers for at least 4 weeks. Partial remission (PR) was characterized by a 30% or greater decrease of the sum of the longest diameters of the target lesions from the baseline, having a non-progressive disease in the non-target lesion, and no emergence of new lesions. The definition of progressive disease (PD) was to have at least a 20% increase in the sum of the longest diameters of the target lesions or the appearance of new lesions. Stable disease (SD) was defined as an insufficient decrease in the tumor size to qualify for PR or an insufficient increase in size to qualify for PD. The toxicity grading was performed in accordance with the WHO toxicity criteria.
5. Statistical Methods
The categorical data were compared using the Fisher’s exact test. The survival curves were constructed using the Kaplan-Meier methods and were compared using the log-rank test. The analysis was performed using the SPSS 8.0 system (SPSS, Inc, Chicago, IL). A p value≤0.05 was considered significant.
RESULTS
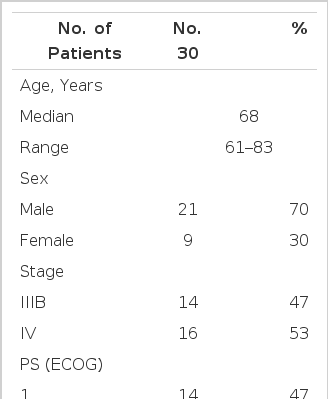
The patient characteristics are listed in Table 1. The median age of the patients was 68 years old (range, 61–83). Fourteen patients had stage IIIB disease, and 16 patients were diagnosed with stage IV disease. The majority of patients (53%) had PS (ECOG≥2). All the patients received at least one cycle of chemotherapy and were assessed for toxicity, and 26 received at least two cycles of therapy and were assessed for the type of their response.
1. Treatment, response and survival
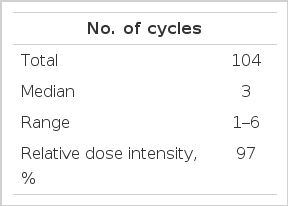
The treatment administration is summarized in Table 2. A total of 104 cycles were administered. The median number of cycles per patient was 3 (range, 1–6). The relative dose-intensity of both paclitaxel and carboplatin was 0.97. A total of 4 patients (13%) were considered to be non-assessable for response: 3 because of voluntary withdrawal, and one because of nausea and vomiting from toxicity after the first course of chemotherapy. Therefore, a total of 26 patients (87%) were assessed for their response.
The response rate was 40% (Table 3). There were no cases of CR’s, and 17% of patients showed the condition of SD and 30% illustrated PD. There was no significant difference between patients with a PS≤2 and patients with a PS≥3 in terms of the response rate (36% vs 50%, p=0.68). Also, these various comparisons illustrate that the response rates were not affected: patients with stage IIIB vs patients with stage IV disease (36% vs 44% p=0.72), patients aged<70 years vs those that have aged≥70 years (42% vs 31%, p=1.00), and histologic type of adenocarcinoma vs histologic type of non-adenocarcinoma (30% vs 45%, p=0.69).
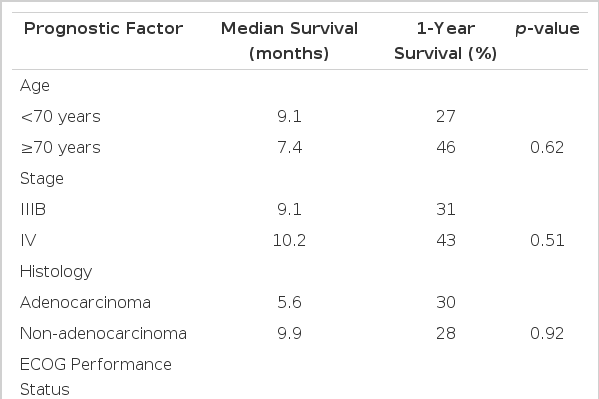
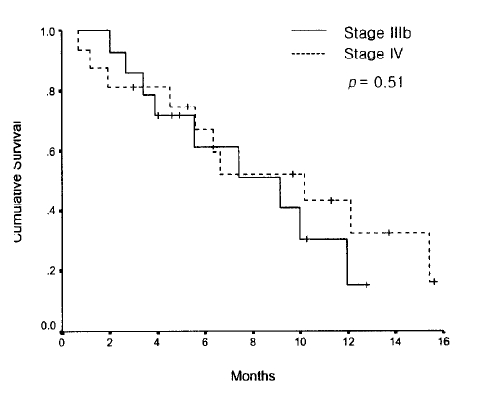
The median overall survival was 9.1 months (95% CI, 4.2 to 14 months), and in all patients, the 1 year survival rate was 31% (Figure 1). Patients with a PS of 1 or 2 had a significantly better survival outcome than patients with a PS of 3 or 4 (1-year survival rate: 40% vs 0%, p=0.02) (Figure 2A, Table 4). However, age, stage, and histological subtype did not affect the overall survival (Figure 2B, Table 4). The median progression-free survival was 7.7 months (95% CI, 3.1 to 12.2 months) (Figure 3). PS, age, stage, and histological subtype did not show any significant differences in terms of the progression-free survival.
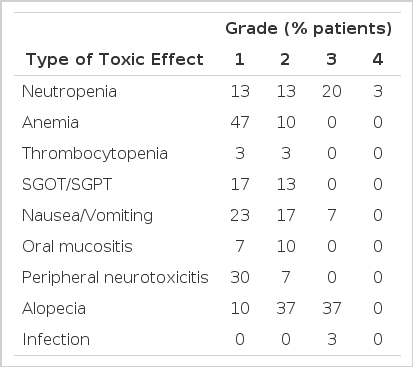
2. Toxicities
The hematological and nonhematological toxicities were evaluated for all the patients, and the results are shown in Table 5. The treatment was generally well tolerated, and hematological toxicities were mild. Grade 3 neutropenia appeared in 23% of patients. There was neither grade 3/4 anemia nor grade 3/4 thrombocytopenia. Nonhematological toxicities were also mild. However, nausea and vomiting occurred frequently (47%) with the grade 3 toxicity occurring in 7% of patients. Alopecia was the most frequent symptom in 37% of patients who had grade 3 toxicity. Peripheral neuropathy and increased transaminase appeared (37%, 30% respectively) without any grade 3 toxicity.
DISCUSSION
This study demonstrated a similar efficacy and a low toxicity of the less intensive combination of paclitaxel/carboplatin inadvanced NSCLC patients older than 60 years of age including those with a poor PS of 3–4. The treatment regimen consisted of paclitaxel 135 mg/m2 by a 1 hour infusion followed by carboplatin at an AUC=6 in a 4 week cycle. The standard regimen for treating advanced NSCLC consists of paclitaxel 175–225 mg/m2 plus carboplatin at an AUC= 6 every 3 weeks, and thus, it is considered more intensive than the regimen under study8, 9). All patients in the two trials had a PS of 0–2. The response rates in these studies were 31.8% and 29%, respectively. The two trials reported median survival times of 9.5 and 9.0 months, and the 1-year survival rates were 37% and 35%, respectively.
However, grade 3–4 neutropenia occurred in 56% of patients, 14% of whom had a neutropenic fever9). On the other hand, this study included 8 patients (26%) with a PS of 3–4. The response rate was 40%, the median overall survival was 9.1 months, and the 1 year survival rate was 31%. Grade 3–4 neutropenia occurred in only 23% of patients. Encouraging results and a favorable toxicity profile was demonstrated, even though this study included elderly patients with a poor PS.
Some clinical factors, such as age and PS, appear to influence the survival in NSCLC patients. Because a large percentage of NSCLC patients are elderly, there have been indications of doubt on whether the use of standard regimens, which are fairly intensive, for the elderly and poor PS patients are effective. Some studies have suggested that elderly patients with advanced NSCLC have poor outcomes10, 11). However, other studies have failed to confirm this or on the flip side, have suggested that older patients have a similar or even superior survival rate12–14). Therefore, it is difficult to say that age is a reliable prognostic factor for these patients. This study also showed no significant differences between those patients < 70 years of age and those = 70 years of age in terms of the response rate and survival. Age was not the only factor considered because PS is generally regarded to be a powerful prognostic factor10–14). In this study, the 1-year survival rate was 40% for the patients with a PS of 1–2, and 0% for the patients with a PS of 3–4 (p=0.02). Toxicity is particularly problematic in patients with a poor PS. Such patients were likely to be more susceptible to adverse events than the patients with a PS of 0 or 1, including adverse events such as death within 30 days from any cause. Since the role of chemotherapy in the treatment of advanced non-small cell lung cancer is supportive and palliative at best, the routine use of platinum-based combination chemotherapy in patients with a poor PS cannot be recommended5).
In conclusion, a less intensive combination of paclitaxel and carboplatin was effective and tolerable in advanced NSCLC patients who were older than 60 years of age and had poor PS.
Acknowledgements
Authors would like to thank Geum Suk Kim, a research nurse, for her assistance in data collection.