A Case of Severe Chronic Active Epstein-Barr Virus Infection with T-cell lymphoproliferative Disorder
Article information
Abstract
Chronic infection with Epstein-Barr virus (EBV) without previous immunodeficiency or immuno-suppressive therapy is relatively rare. Severe chronic active EBV (SCAEBV) infection was reported for the first time in 1984 as ‘chronic mononucleosis syndrome’, and diagnostic criteria were proposed. It is characterized by clinical features including fever, severe hepatosplenomegaly, lymphadenopathy, hematologic features such as anemia and thrombocytopenia, and elevated antibody titers to EBV. We experienced a 21-year-old woman who initially presented with fever and chronic fatigue; however, no definite diagnosis could be made at the time of admission. Three months after the initial admission, there was evidence of only splenomegaly and the patient had persistent, multiple, paraaortic lymphadenopathies in abdominal CT. Diagnostic splenectomy was performed, and SCAEBV infection with T-cell lymphoproliferative disorder was ultimately diagnosed.
INTRODUCTION
Severe chronic active Epstein-Barr virus (SCAEBV) infection is a rare disease with a high mortality and morbidity with life-threatening complications, such as virus associated hemophagocytic syndrome, interstitial pneumonia, lymphoma, coronary artery aneurysms, and central nervous system (CNS) involvement1–5). In 2001, Lee et al.6) reported a case of SCAEBV with hepatic involvement in Korea. Herein, we report a case of SCAEBV with T-cell lymphoproliferative disorder confirmed by diagnostic splenectomy.
CASE REPORT
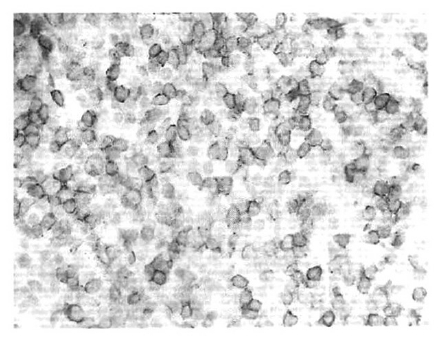
A 21-year-old woman was admitted to our hospital with the chief complaint of febrile sensation developed 6 months previously. Initially, she was admitted to the department of rheumatology and went through many examinations for evaluation of fever of unknown origin. However, no definite diagnosis was made, except for splenomegaly and multiple, small-sized, paraaortic lymphadenopathy observed in abdominal CT, and elevated liver function test. Therefore, she was transferred to the department of hemato-oncology for further evaluation of fever, splenomegaly, and lymphadenopathy. Her past medical history and family history were unremarkable. On admission, her temperature was 38°C, pulse 90/min, respiration 18/min, and blood pressure 120/80 mmHg. The fever pattern showed early morning high peak, and normal body temperature was observed more than once in a day. Physical examination revealed that she was a chronically fatigue woman in no acute distress, and had an anemic conjunctiva. There was no evidence of cervical lymphadenopathy or abnormal findings in chest and bowel auscultation. Splenomegaly was palpable about 3-finger-breadths in the left upper quadrant, but there was no specific tender point. The laboratory evaluations were as follows: white blood cell count 2,400/μL (neutrophil 51.1%, lymphocyte 37%, monocyte 10.6%, basophil 0.7%, eosinophil 0.6%), hemoglobin 7.7 g/dL, platelet count 147,000/μL, MCV 74.9 fL, and MCHC 32.2 g/dL. Serum iron level, total iron binding capacity and ferritin were within normal ranges. Serum electrolyte was also within the normal range, but albumin level was slightly below normal at 3.0 g/dL, cholesterol at 82 mg/dL, and AST/ALT was increased to 103/124 U/L. C-reactive protein (CRP) was increased to 4.3 mg/dL (normal: 0.1–0.8 mg/dL), prothrombin time percentage was 81% (normal: 0–70%), and partial thromboplastin time was 34 sec (normal: 26 sec). Hepatitis B surface antigen was negative with surface antibody positive. Urine analysis was within normal limits. Stool occult blood test showed negative findings. Chest X-ray, simple abdomen X-ray and electrocardiography were normal. Peripheral blood smear showed normocytic normochromic anemia pattern and revealed anisocytosis and poikilocytosis, but there was no evidence of malaria. Blood, urine and sputum culture were all negative. ANA titer was 1:40, with speckled type. ANCA, lupus anticoagulant, anti-cardiolipin antibody, and anti-double-stranded DNA antibody were all negative. Complement 3 (C3) level was 147.0 mg/dL (normal: 79–152 mg/dL), and complement 4 (C4) 28.2 mg/dL (normal: 16–38 mg/dL). EBV (VCA) Ig M was negative, and Ig G to EBV was positive. Ig M to cytomegalovirus (CMV) was negative, and Ig G to CMV was positive. β2-microglobulin was 2.3 mg/L (normal: 0–2.4 mg/L), and serum and urine immuno-electrophoresis were within normal limits. Bone marrow section was normocellular (50%) for her age with increased megakaryocytes. The myeloid series and the erythroid series were in normal proportion. Abdominal CT showed multiple, small-sized (below 1 cm), paraaortic, aortocaval, retrocaval lymphadenopathies, splenomegaly, and small amounts of ascites. We could not find a definite origin of fever, thrombocytopenia, lymphadenopathy and splenomegaly in spite of many examinations after admission. Therefore, she was transferred to the department of general surgery to undergo diagnostic splenectomy. Operative findings revealed an enlarged spleen, measuring 24×13.5×6 cm and weighing 930 g. The outer surface was focally attached with fibrous tissue, but the capsule appeared intact and tense. On section, the cut surface was markedly congested with oozing, dark brownish blood. There were prominent white pulps on the cut surface. Microscopically, the spleen was markedly congested with dilatation of sinusoidal spaces. There were a few foci of nodular aggregates around the white pulp (Figure 1), which was composed mostly of atypical CD3 positive T lymphocytes, some of which were positive for EBV latent membrane protein (LMP) (Figure 2), P53, and KI-67 positive. B-cell markers (CD20 and CD79a) and CD56 were negative. The lymph node showed widening of the interfollicular T zone with infiltration of atypical large CD3, P53, and KI-67 positive T lymphocytes. Some of these were EBV LMP-1 positive. These cells were negative for CD56, CD30 and CD15. EBV polymerase chain reaction (EBV-PCR) with EBNA 1 primer showed a strong positive band at 138bp (Figure 3). In TCRγ gene rearrangement, PCR amplification using primers Vγ 1–8A, B/Jγ, Jγ 2, Vγ 10/Jγ 1, and Jγ 2 showed monoclonal bands by 2% gel electrophoresis (Figure 4). These findings indicated the monoclonal nature of the lesion. After splenectomy, EBV (VCA) Ig G titer was extremely high (1:5120), confirming the final diagnosis of SCAEBV. The final diagnosis was made as SCAEBV infection with T-cell lymphoproliferative disease. She received CHOP (cyclophosphamide 750 mg/m2, adriamycin 50 mg/m2, vincristine 1.4 mg/m2, and prednisone 100 mg/day) chemotherapy. After starting CHOP chemotherapy, the fever was subsided. She received 6 cycles CHOP chemotherapy at postoperatively x months and at the time of writing had continued follow-up at our out patient department for 3 months without any definite symptoms.
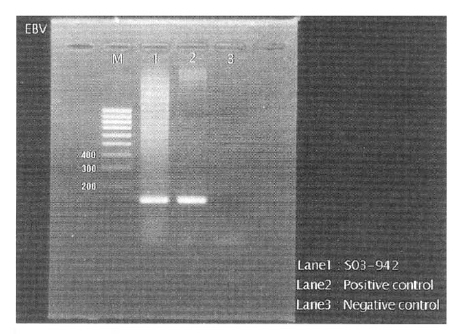
EBV PCR with EBNA 1 primer showing strong positive band at 138bp.
Lane M: size marker (100bp ladder, MBI)
Lane 1: patient sampleLane 2: positive control
Lane 3: negative control

TCR gene rearrangement using V1-8A, B/J1, J2, V10/J1, and J2 showing monoclonal bands by 2% gel electrophoresis.TCR gene rearrangement using V9, B/J1, J2, V11/J1, and J2 showing no monoclonal band.
Lane M: size marker (100bp ladder, MBI)
Lane 1, 4, 6, 8: patient sample
Lane 2: positive control (V1-8A, B/J1, J2)
Lane 3, 5, 7, 9: negative control
DISCUSSION
EBV is a ubiquitous human herpes virus that establishes lifelong latency and transforms B-cells7, 9). Infection with EBV is generally asymptomatic in young children; however, infection in adolescence results more commonly in the acute infectious mononucleosis syndrome7, 10). Chronic active EBV (CAEBV) infection is an uncommon outcome of EBV infection, and may present as a waxing and waning or fulminant syndrome7). Patients with this disease have no evidence of any prior immunologic abnormalities or of any other recent infection that might explain their condition10, 11). CAEBV infection is a disease with high mortality, high morbidity and life-threatening complications, such as virus associated hemophagocytic syndrome, interstitial pneumonia, lymphoma, coronary artery aneurysms, and CNS involvement1–5).
In 1948, before the discovery of EBV, an undefined illness characterized by prolonged fever, malaise, lymphadenopathy and hepatosplenomegaly was described by Issacs12). The illness was similar to infectious mononucleosis (IM), but the symptoms persisted for a longer period of time. In 1975, Horwitz et al13). described mild or moderate cases with such clinical manifestations, and DuBois et al.8) proposed the term ‘Chronic mononucleosis syndrome’ for such cases in 1984. Straus11) defined 3 main criteria of CAEBV infection in 1988, and in 1991 Okano et al.1) proposed the following new diagnostic criteria of severe CAEBV: (1) clinical findings including intermittent fever, lymphadenopathy, and hepatosplenomegaly; (2) hematologic findings including anemia, thrombocytopenia, lymphocytopenia, lymphocytosis, neutropenia, and polyclonal gammopathy; (3) virological elevated antibody titers and positivity for antibodies to EBV-related antigens (VCA IgG, = 5,120; VCA IgA, positive; EA [D] IgG, = 640; EA [D] IgA, positive; and EA [D] and EA [R] IgG, = 640) and/or detection of EBV genomes in affected tissues; and (4) chronic illness which cannot be explained by other known disease processes. Clinical and hematologic findings of our patient satisfied the criteria of SCAEBV; she had intermittent fever, multiple, small-sized, paraaortic lymphadenopathy, splenomegaly, and thrombocytopenia. Diagnostic splenectomy was performed, and EBV genomes were identified in the tissue by immunohistochemistry and PCR, which led to a final diagnosis of SCAEBV with T-cell predominant-lymphoproliferative disorder. After splenectomy, the diagnosis was confirmed by the extremely high EBV (VCA) Ig G titer (titer of 1:5120).
Based on the cellular target of EBV, CAEBV can be divided into T- and NK-cell cell subtypes1, 14). Titers of anti-EBV related antibodies are higher in the T-cell than in the NK-cell category. Although mortality is higher when the T cell is the target, chromosomal abnormalities are more frequent in the NK cell disease. T-cell type CAEBV is associated with the development of lymphoma14). SCAEBV is an undefined illness, and therefore, when the underlying diseases are clarified, the diagnosis may be altered back to the original disease. For example, EBV genome positive Burkitt’s lymphoma, nasopharyngeal cancer, and Hodgkin’s disease have high anti-EBV antibody titers; therefore, careful pathological examinations can differentiate CEBV or SCAEBV and EBV genome-positive malignancies. Autoimmune disease and known infectious disease often result in active EBV status; however, they are differentiated from CAEBV or SCAEBV by careful clinical and laboratory findings9).
Although there have been many therapeutic trials for SCAEBV, no conclusive beneficial effects have been shown. Vidarabine therapy has recently been reported as a therapeutic choice to control SCAEBV15). As immunomodulating therapy, IL-2 was reported to be effective for SCAEBV16), and anticancer chemotherapy including etoposide was also given to certain patients with SCAEBV, but without clinical improvement17). A trial of anti B lymphocyte (anti-CD21 and anti-CD24) antibodies has shown some promise in the treatment of EBV-related B-cell lymphoproliferations in transplant recipients18), and blood stem cell transplantation has also been performed in cases refractory to other therapies19). The prognosis of SCAEBV remains very poor. Recently, Kimura et al.20) reported that patients with CAEBV with late onset of disease, thrombocytopenia, and T-cell infection showed significantly poorer prognosis. In the present study, the patient received 6 cycles of CHOP chemotherapy after splenectomy, and remained on follow-up for 3 months at our outpatient department without any definite symptoms. The present case should assist in the diagnosis of other cases of SCAEBV with T-cell lymphoproliferative disorder.
CONCLUSION
We described a case of SCAEBV with T-cell lymphoproliferative disorder which was diagnosed by means of diagnostic splenectomy, and also reviewed the literature on this unusual disease. Finally, although there have been many reports describing SCAEBV in Japan, this is only the second such case report in Korea until now. We must suspect SCAEBV with lymphoproliferative disorder in patients with fever of unexplainable origin and chronic fatigue.