A Large Pedunculated Left Ventricular Thrombus with Recurrent systemic Thromboembolism in a Young Man
Article information
Abstract
We present the case of a young man who has suffered from recurrent systemic thromboembolism since he developed an acute interior wall myocardial infarction at the age of 27. A large elongated, pendunculated left ventricular thrombus was found by two dimensional echocardiography and was successfully removed by open heart surgery.
INTRODUCTION
The mural thrombus in the left ventricle is known to develop in between 20 and 40% of patients1,2) and systemic arterial thromboembolism develops in about 3 to 5% of patients after acute myocardial infarction.3)
The pedunculated left ventricular thrombus is very rarely reported, and its nature is not well defined. This kind of lesion may be more prone to cause systemic thromboembolism than the mural thrombus. Recently we had encountered a young man with a very unusual history. He had developed an acute inferior wall myocardial infarction at the age of 27 and had suffered from recurrent systemic thromboembolism since then. A large, pedunculated, left ventricular thrombus was found by two-dimensional echocardiography and successfully removed by open heart surgery.
CASE
On Feb. 3, 1982, a 33 year old man, an industrial worker, was admitted to the general surgery department of Korea University Hospital because of intermittent claudication and longstanding ulceration of the right leg, which developed following acupuncture on his right leg. The perfusion was very poor and the arterial pulses were not palpable over the femoral arteries and down either leg. An aorto-femoral angiogram revealed occlusion of the right femoral artery with patent anterior tibial, posterior tibial, and peroneal arteries, and the occlusion of the left internal iliac artery with collateral arteries.
For the purpose of limb salvage, embolectomy and right femoro-tibial saphenous vein bypass graft surgery was attempted. There was no evidence of atherosclerosis in the leg vessels. Twelve hours postoperatively, the bypass graft stopped functioning. Reoperation was done and thrombectomy was carried out, but the patient finally had to undergo above-knee amputation on the right leg at a later date. During this admission, the patient experienced multiple episodes of precordial chest pain and an episode of colicky left flank pain.
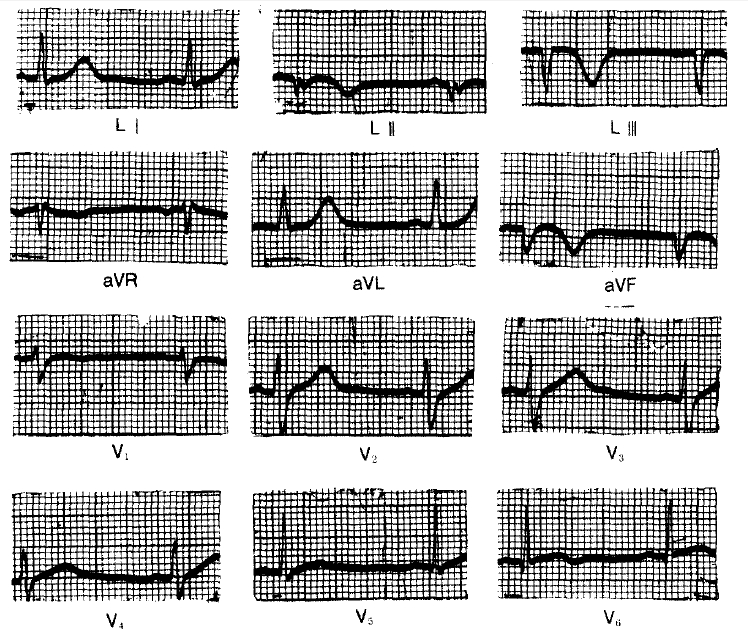
According to the past medical history, he was first admitted to Korea University Hospital because of severe chest pain, which awakened him early in the morning of July 23, 1976. The electrocardiogram on admission showed changes compatible with an acute inferior wall myocardial infarction (Fig. 1), the peak SGOT was 230 IU L and the peak LDH was 1500 U ml (Wrobleski). The hospital course was uneventful except for the intermittent episodes of abdominal pain. He was discharged on Aug. 16, 1976.
Between Aug., 1976 and Feb., 1982, the patient had to be amitted to the hospital three times. The first time was for the sudden development of dizziness, the second time for abdominal pain due to ileus, and the third time because of chest tightness and abdominal pain.
He had had an appendectomy at the age of 13 and had contracted typhoid fever at 14. According to his history his father and younger brother had hypertension. He smoked about two packs of cigarettes per day and consumed a little alcohol.
The chest X-ray revealed mild cardiomegaly with a prominent left ventricle. Occasional premature ventricular contractions were noted on the twelve-lead electrocardiogram. The hemoglobin was 14.0 gm dl; hematocrit 42%: ESR 30 mm hr: WBC 12,250 with 50% of segs, 22% of lymphs, 10% of monos, and 12% of eosinophils. The platelet count was 258,000 mm3. The bleeding time and the coagulation time were normal. The serum cholesterol was 167 mg dl and the triglyceride was 55 mg dl. The lipoprotein electrophoresis revealed that α-lipoprotein was 22.7%, prebeta 28.6%, and beta 48.7%, the RA factor was negative. The protein electrophoresis revealed albumin 50.9%, α1 3.2%, α2 10.9%, β13.8%, and γ21.2%. The patient was discharged on Apr. 14, 1982.
On July 3, 1982, the patient was admitted to the hospital because of abdominal pain. The abdomen was tender and distended, and the bowel sound was diminished. Under the impression of mesenteric artery thromboembolism heparinization and subsequent coumadinization was done. To rule out the possibility of embolization from the heart two dimensional echocardiography was done. This reveled a large, pedunculated mass which had its base in the apex of the left ventricle and its end freely movable in the left ventricular outflow tract (Fig. 2). The mitral valve leaflets were seen striking the mass during its motion. The mass was 7×3 cm. The mass reflected echoes which were dense compared with the lighter echoes from the myocardium. The outline of the mass was rather well defined and several daughter masses were noted to be budding from the main mass. The left ventricle was only slightly enlarged in size, and the apex and the distal septum were noted to be akinetic. Under the impression of either a left ventricular thrombus or a myxoma open heart surgery was performed on July 9, 1982.
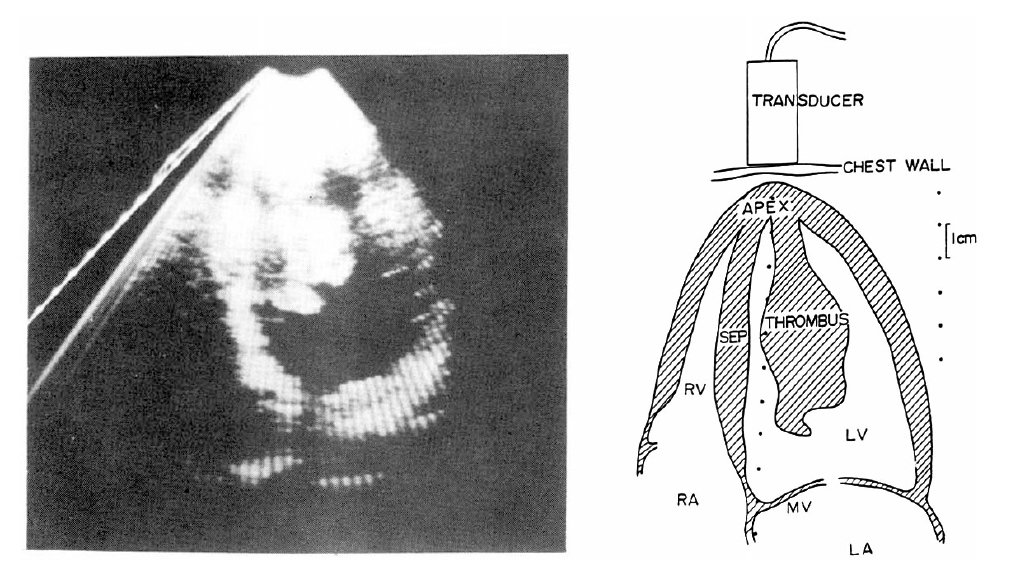
Echocardiogram Apical four chamber view showing pedunculated left ventricular thrombus. Schematic diagram of this thrombus is shown on right.
In the operative field, the left ventricular apex was noted to be fibrotic and moderately adhered to the regional pericardium in an area of about 3×5 cm2. The left ventricle was opened through the infarcted area. The organized thrombus -like mass, which was pedunculated from the inner surface of the apex and intermixed with the muscular trabeculae was removed en bolc with the central infarcted apical wall to preserve the total continuity of the mass. The left ventricular wound was closed with heavy, interrupted, cross suture and pledgeted dacron flet velour.
The gross specimen measured 7.0×4.5×2.7cm and weighed 35 gm (Fig. 3): On pathologic examination the mass was confirmed to be a thrombus. The surface of the mass was irregular and grayish-pink colored with multiple hemorrhagic foci. On the cut surface could be seen whitish-yellow-to-pinkish-brown, soft tissue with interlacing bundles. The microscopic examination revealed fibrinous lamellated eosinophilic material mixed with coagulated RBSs, WBCs, platelets, cholesterol clefts, and focal calcifications. At the periphery of the mass, fibroblastic proliferation was seen, sections from the right autricle and the left ventricle revealed the coagulative necrosis of myocardial fibers and fibroblastic proliferation.

Anterior view of the thrombus taken from left ventricle. Left end of the thrombus was connected to left ventricular apex and right end was free-floating in left ventricular outflow tract. Thrombus measured 7.0×4.5×2.7 cm and weighed 35 gm.
On July 23, 1982, he developed impending gangrene of the left leg. Thromboembolectomy of the left femoral artery was done without success. Finally, he had to undergo the above knee amputation on the left leg. During this admission, the patient experienced squeezing precordial pain and intermittent abdominal pain.
On Aug. 30, 1982, factor VIII concentration was 200% and factor V 100% of the normal control. The euglobulin lysis time was 3 hours. FDP was less than 10 μg/dl. The fibrinogen was 407 mg/dl. The serum antithrombin III level was within normal range. The platelet aggregation test revealed increased aggregability with ADP (patient, 91.3%; control, 77.5%), epinephrine (patient, 82.5%; control, 72.5%), and collagen (patient, 81.3%; control, 70.0%).
The two-dimensional echocardiography repeated on Oct 6, 1982 revealed no evidence of recurrence of thrombus in the left ventricle. The patient was discharged on Now. 16, 1982. He has been followed in the outpatient clinic and has been doing reasonably well so far, except for occasional mild left precordial chest pain. A thallium-201 myocardial imaging was done on June 29, 1983, by injecting 2 mCi of thallium-201 intravenously following the intravenous injection of 7 mg of dipyridamole. It showed a perfusion defect of the inferior wall, the site of the previous myocardial infarction. The perfusion defect did not improve with the delayed images taken four hours later.
DISCUSSION
A left ventricular thrombus is most frequently observed after an acute transmural myocardial infarction. Most of the time it has been seen in association with a left ventricular aneurysm.
The thrombus has been observed in the left ventricle in the other disease states, such as cardiomyopathy, ventricular aneurysm from trauma or a hypercoagulable state.
A left ventricular thrombus is found in about 20 to 40% of autopsy patients following an acute myocardial infarction1), and in about 40 to 60% of patients, undergoing left ventricular aneurysmectomy.4) In Asinger’s prospective study in which a two-dimensional echocardiography was done on every patient serially after acute myocardial infarction, left ventricular thrombus was found in 12 of 70 patienys(17%).2) Despite this not uncommon incidence of left ventricular thrombus, systemic arterial thromboembolism has been observed in only 3 to 5% of patients after acute myocardial infarction.3,4)
Most of the left ventricular thrombi are seen after acute myocardial infarction as mural thrombi, and a large, elongated, pedunculated, left ventricular thrombus has been rarely reported. Recently, McManus and his associates5) reported a 54-year-old man with problems quite similar to those of our patient. The patient suffered an occlusion of the distal abdominal aorta 6 months after an acute posterior wall myocardial infarction and was found to have a large, elongated, pedunculated thrombus in the left ventricle. This was confirmed at autopsy. McManus reported that in the over 500 previous autopsy cases done in his hospital, he could find only one other case with similar features of left ventricular thrombus. The patient was a 20-year-old man who had developed an anterolateral wall myocardial infarction and an apical aneurysm alter chest trauma and later developed a large, pedunculated, left ventricular thrombus.6)
Our patient has, many unusual features. He developed an acute inferior wall myocardial infarction at the age of 27, which is quite unusual, especially in Korea, where the incidence of myocardial infarction is generally known to be much less frequent compared with the incidence in occidental countries. On the pathologic specimens of the amputated legs the atherosclerotic changes were rather scanty. From the history and the clinical course, it is quite likely that our patient has had multiple systemic arterial thromboemboli. The patient had an akinetic area involving the apex of the heart and the distal septum and the size of left ventricle was only mildly enlarged. The lack of gross aneurysmal sag might have favored the formation of the pedunculated thrombus rather than a mural thrombus because of the presence of the contractile wall adjacent to the akinetic wall.3)
Since patient did not have coronary angiography the pathologic anatomy of the coronary arteries is not known. The 201thallium myocardial images showed the persistent perfusion defect in the inferior wall which is compatible with the old inferior wall myocardial infarction, it might also be thought that the thrombus was attached first in the left ventricle and the coronary artery embolism followed. Although the platelet aggregation, factor V, factor VIII, and fibrinogen showed increased levels, it is difficult to evaluate the significance of these findings because these were measured postoperatively and they are also nonspecific. The extensive studies searching for lipid abnormalities and collagen diseases did not reveal any unusual features.