Helicobacter Pylori Infection and Serum Pepsinogen I Concentration in Peptic Ulcer Patients: Effect of Bacterial Eradication
Article information
Abstract
Objectives:
In order to test the hypothesis that H. pylori infections in the gastric antrum increase pepsinogen I release, fasting serum pepsinogen I concentrations were compared in peptic ulcer patients with and without H. pylori infection. A randomized prospective study was performed to determine whether the increased serum pepsinogen I concentrations associated with H. pylori infection respond to treatment that eradicates H. pylori.
Methods:
Fasting serum pepsinogen I concentrations were measured by RIA in 736 patients with endoscopically and histologically confirmed benign peptic ulcer with and without H. pylori infection. Out of 511 patients with H. pylori infection, 110 patients (group 1) were randomly selected and were treated with metronidazole and tripotassium dicitrato bismuthate combined with ranitidine and antacid, and 97 patients (group 2) were treated only with ranitidine and antacid. The third group, 54 patients free of H. pylori infection, was designed to evaluate the influence of H2-receptor antagonist and antacid on the change of pepsinogen I. Fasting pepsinogen I concentration and H. pylori status were compared before and after the treatment.
Results:
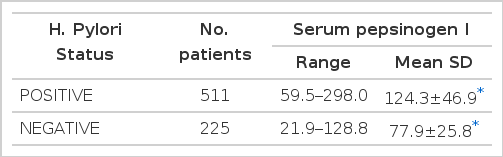
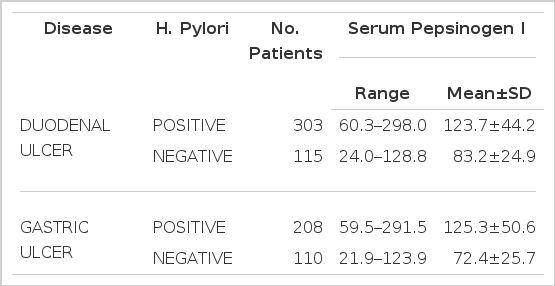
Patients infected by H. pylori (gastric ulcer 208, duodenal ulcer 303; total 511) had significantly higher fasting serum pepsinogen I concentrations than H. pylori negative patients (gastric ulcer 110, duodenal ulcer 115: total 225). Mean pepsinogen I level of the former was 124.3±46.9 and that of the latter was 77.9±25.8 ng/ml. (p<0.0001) The difference in serum pepsinogen I concentrations according to the location of ulcer crater was significant only in non-infected subjects e.g., mean pepsinogen I level H. pylori-negative gastric ulcer was significantly lower than that of H. pylori-negative duodenal ulcer patients. H. pylori was eradicated in all the patients who had received antibacterial therapy for 4 weeks and serum pepsinogen I concentrations were significantly decreased from 129.8+43.0 to 82.4±24.0 ng/ml after eradication of the organism. (p<0.0001) In contrast, H. pylori-positive patients who had not received antibacterial therapy were still infected at the completion of the study and there was no significant change in the serum pepsinogen I concentrations after the treatment (120.8±40.9 vs 126.3± 40.4 ng/ml). (p>0.57) None of the patients who were initially H. pylori-negative has been reinfected during the period of the study and their serum pepsinogen I concentrations were not changed. (pre-treatment value 75.1±8.0 ; post-treatment value 77.3±24.5 mg/ml)(p<0.75) Four-to six-week therapy of H2-receptor antagonist and antacid did not exert any influence on serum pepsinogen I concentrations.
Conclusions:
On the basis of our results, we have confirmed that the chronic infection of H. pylori of gastric antrum in peptic ulcer patients causes increased pepsinogen I release into the circulation, and eradication of the organism results in significant fall in serum pepsinogen I concentrations.
INTRODUCTION
The concentration of pepsinogen I (PG I) in serum is raised in both gastric (GU) and duodenal ulcer (DU) patients1–6) and correlates positively with the relapse rates of DU7). Helicobacter pylori (H. pylori) infection of the gastric antrum is now regarded as one of the important pathogenetic factors for developing the peptic ulcer diseases, and eradication of the infection markedly lowers the ulcer relapse rate.
Recently, it has been suggested that, in humans and animals, serum PG I secretion rates increase with age as does the prevalence of H. pylori infection8–11). However, there is no consensus on how the mechanism interact with H. pylori infection and hyperpepsinogenemia. Oderada et. al12), reported that eradication of H. pylori infection in children with various gastrointestinal disorders results in 25% reduction in the serum PG I concentration. It has become apparent that many of secretory abnormalities in DU, such as hypersecretion of acid, pepsin and gastrin, are to a large extent secondary to H. pylori infection and its associated gastritis6,13). The chronic inflammation of the gastric antral mucosa induced by H. pylori might increase leakage of the zymogen into the circulation. In addition, H. pylori infection raises the plasma gastrin concentration2–4,12,14–16 and the trophic action of this hormone may increase the chief cell mass and thereby the PG I synthesis.
We have examined the influence of H. pylori infection on serum PG I concentration in patients with peptic ulcer. In addition, we have investigated the extent of any changes of serum PG I concentration after eradication of H. pylori.
MATERIALS AND METHODS
1. Patients
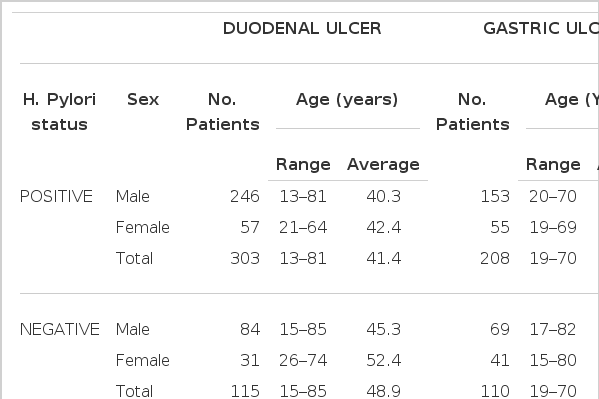
The study population was made up of 736 patients with endoscopically and histologically confirmed benigh GU and DU. Five-hundred-eleven patients were infected with H. pylori and the remaining 225 patients were negative for the organism. Among H. pylori-positive patients, 303 patients were DUs (male 246, mean age 40.3: female 57, mean age 42.4) and 208 were GUs (male 153, mean age 45.7:female 55, mean age 52.1). H. pylori negative-patients were consisted with 115 DU patients (male 84, mean age 45.3, female 31, mean age 52.4) and 110 GU patients (male 69, mean age 50.7, female 41. mean age 52.6) (Table 1).
2. Methods
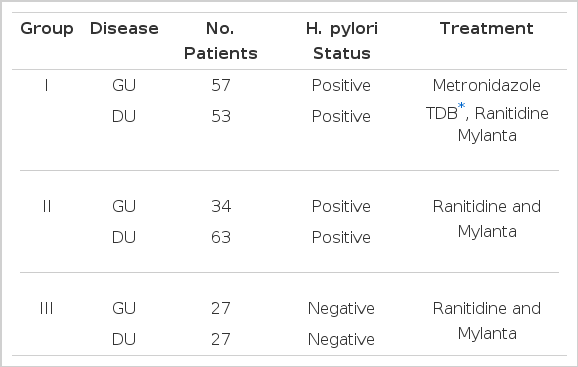
A blood sample was collected after an overnight fast for the measurement of serum PG I concentration. After this, gastro-duodenal endoscopy was performed with either the Fujinon EVG-FP or Olympus (Japan) endoscope. H. pylori infection was confirmed in each by identification of the organism by Gram staining, culture and urease testing, and by Giemsa or Warthin-Starry silver stainging of biopsy specimens of the antral mucosa. Serum PG I concentrations were measured by the radioimmunoassay techinique using the [125I] Pepsinogen RIA kit (Pepsik P2560, Sorin Biomedica, Italy) and each examination was duplicated. On the basis of H. pylori status and therapeutic modalities, 261 patients were divided randomly into three different groups (Table 4). Patients with poor compliance or previous antibacterial therapy against H. pylori were excluded. The first group consisted of 110 H. pylori-positive patients (57 GUs and 53 DUs) and was treated with metronidazole 250 mg qid., tripotassium dicitrato bismuthate (TDB) 120 mg qid., designed to eradicate H. pylori in addition to ranitidine 300mg at bed time and antacid 100ml qid. The second group of 97 H. pylori-positive patients (GU 34, DU 63) was treated only with ranitidine and antacid. The third group(GU 27, DU 27) free of the infection of H. pylori was treated with ranitidine and antacid. The third group was designed to evaluate the effect of H2-receoptor antagonist and antacid on the serum PG I concentration and H. pylori status. Metronidazole and TDB were given for 4 weeks and ranitidine and antacid were given for 4 to 6 weeks according to the size of ulcer crater. Both healing of active ulcer crater and confirmation of eradication of H. pylori were achieved by repeating the endoscopy and antral biopsies.
On the day of follow-up endoscopy, when ulcers were completely healed, repeated fasting blood samples were obtained for estimation of serum PG I level and the H. pylori status was reassessed by means of microbiologic and histologic examination of antral biopsies.
STATISTICAL EVALUATION OF RESULTS
Result were expressed as the Means±SD. Student’s t-test was used to determine the significance of difference between means, with differences giving a p value less than 0.001 being considered significant.
RESULTS
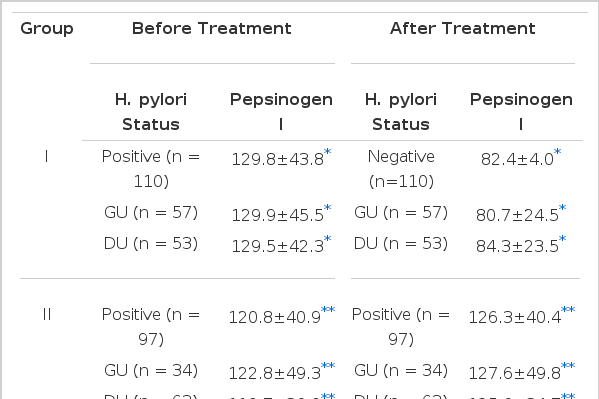
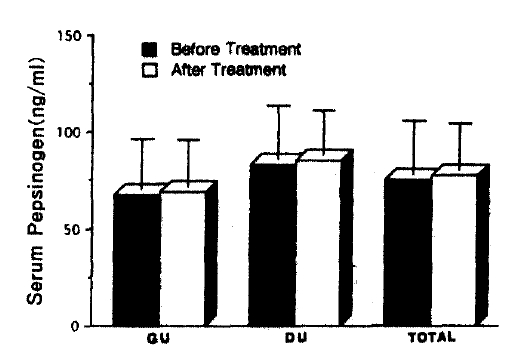
All of 736 patients studied, who had either GU or DU, were effectively treated with ranitidine and antacid for 4- to 6-week treatment without any significant side reactions and active ulcer craters were completely healed in all patients on the follow-up endoscopy. After 4-week treatment with metronidazole and TDB, none of the first group patients, who were infected by H. pylori initially, had antral H. pylori both in microscopic and histologic examination of repeated biopsy specimens (Table 4 & 5). However all the second group patients, who were also H. pylori positive at the beginning of the study, and were given only ranitidine and antacid, were still infected by the organism on repeated examination even though ulcers were completely healed (Table 4 & 5). These data suggest that H2-receptor antagonist and antacid do not have any antibacterial action against H. pylori and the 4-week combination therapy of metronidazole and TDB is highly effective for eradication of H. pylori from the antrum. Among the third group patients, initially free of H. pylori infection, none was infected by the organism during the study (Table 4 & 5). Patients infected by H. pylori (511 patients ; GU 208, DU 303) had significantly higher fasting serum PG I concentrations than patients negative for the organism (225 patients: GU 110, DU 115)(range 59.5–298.0 mean 124.3±46.9 ng/ml vs range 21.9–128.8 mean 77.9±25.8 ng/ml, respectively). (p<0.0001)(Table 2 & Fig. 1). The difference in serum PG I concentrations according to the location of ulcer crater was also evaluated. H. pylori positive patients, either GU or DU, had significantly higher PG I levels in serum compared to H. pylori negative patients. (p<0.0001, respectively) (Table 3). In cases of non-infected patients, serum PG I level of GU patients was significantly lower than DU patients (72.4 ± 25.7 vs 83.2 ± 24.9 ng/ml). (p<0.0001)(Table 3). In contrast, the difference between H. pylori-positive GU and H. pylori-positive DU was not significant (125.3 ± 50.6 vs 123.7±44.2 ng/ml(p>0.5) (Table 3). In the 110 patients with successful eradication of H. pylori, there was a significant fall in the fasting serum PG I concentration from 129.8±43.0 ng/ml to 82.4 ±24.0 ng/ml after treatment (p<0.001)(Table 5. Fig. 2). In contrast, fasting serum PG I concentrations of the second group patients whose H. pylori infections were not eradicated were increased even though the difference was not statistically significant (pre-treatment value, 120.8±40.9 ng/ml and post-treatment value: 126.3±40.4 ng/ml)(p>0.5) (Table 5, Fig. 3). In the third group of H. pylori negative 54 patients, there was no change in the fasting serum PG I concentration after the healing of ulcers, with pre-treatment value being 75.1 ±28.0 mg/ml and post-treatment value being 77.3±24.5 ng/ml (p>0.1)(Table 5, Fig. 4).

Fasting serum pepsinogen I concentration between H. pylori positive and negative patients. p<0.0001.
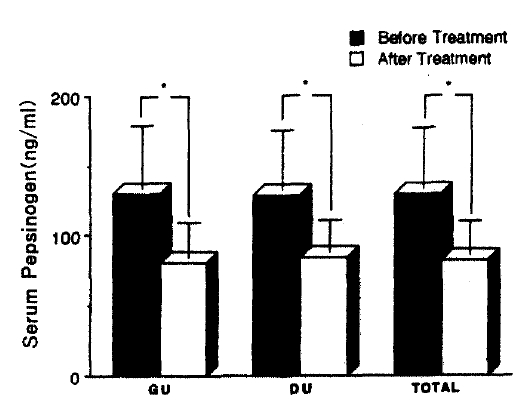
Changes in serum pepsinogen I concentrations before and after treatment in group I. Group I are HP(+) patients treated with metronidazole, TDB. ranitidine and mylanta. The asterisk indicates less than p<0.0001.

Changes in serum pepsinogen I concentration before and after treatment in group II patients. Group II are HP(+) patients, not receiving antibacterial therapy.
DISCUSSION
It is well known that a high serum pepsinogen level is both a feature of established duodenal ulcer and a risk factor for developing duodenal ulcer. In addition, it is also true that in some families with a prominent history of duodenal ulcer an elevated serum PG I level is inherited as an autosomal dominant trait and is a subclinical marker of susceptibility for developing duodenal ulcer disease. Increased serum PG I is also present in patients with gastric ulcer, but there has been no evidence that hyperpepsinogenemia is a risk factor of gastric ulcer disease1–6). Evidences have been accumulated that various gastric secretory abnormalities associated with peptic ulcer disease are to a large extent secondary to H. pylori infection and its associated gastritis. Even more interesting is the finding that somatostatin deficiency of the gastric mucosa induced by H. pylori infection appears to play an important pathogenetic role in hypersecretion of gastrin and acid in DU patients17,20). Before evaluating effects of H. pylori infection on the change of serum PG I concentration, we have examined any possible influences of 4- to 6-week treatment of H2-receptor antagonist (ranitidine) and antacid (mylanta) on serum PG I levels. Patients were completely tolerant of the therapy and there were no side effects. There was no change in fasting serum PG I concentrations between the pre-treatment and the post-treatment period (75.1 ± 28.0 ng/ml vs 77.3 ± 24.5 ng/ml). These observations suggests that short-term treatment with either H2-receptor antagonist or antacid does not have an influence on serum PG I concentrations.
In this report, we found that serum PG I was significantly elevated in patients with peptic ulcer diseases with H. pylori infection compared with those without H. pylori infection. This observation is consistent with previous reports on PG I elevations in patients with peptic ulcer1–9,11,17–21). Interestingly, when comparing the difference of fasting serum PG I levels according to the status of H. pylori infection and the location of ulcer crater, the rate of increment was significantly higher in GU patients than in DU patients (73.1% in GU patients vs 48.7% in DU patients) (Table 3). This study also showed that eradication of H. pylori infection was accompanied by a significant fall in the serum PG I concentrations in both GU and DU patients. In contrast, serum PG I concentrations have increased in patients showing continuously positive for H. pylori and have not been changed in those patients without H. pylori infection in both the beginning and the end of study. Our results are similar to those of previous reports13,15,22,23). Therefore, our observations that reversible hyperpepsinogenemia I strongly suggests that the increased circulating PG I levels in both GU and DU subjects must be resulted from this bacterial infection. The mechanism of the increased circulating levels of the PG I in peptic ulcer patients with H. pylori infection is still unclear. The effect of acid inhibiting therapy could be responsible for the change in serum PG I concentration after eradication of H. pylori infection. Jansen et al.24) and Biemond et al.25) reported that serum PG I level was reversibly increased during the administration of omeprazole. This possibility, however, is excluded by our findings that, while in the subjects of our study who were treated with only H2 blocking drug and antacid, there were no falls in PG I concentratins. Chittajallu et al.26) suggested that the increased serum PG I concentration could be the end result of H. pylori-induced gastritis. Their hypothesis is that the H. pylori-associated gastritis usually involves the body of the stomach to a significant extent and could result in damage to the chief cells and the mucous neck cell, rusulting in leakage of zymogen into the circulation. And it is also possible that the gastritis increases the permeability of the gastric epithelial surface, enabling back-diffusion of PG I before converting pepsin by the gastric acid.
Studies have shown that the serum PG I concentration correlates with the chief cell mass, basal pepsin output and pentagastrin-stimulated gastric acid and pepsin output. And, it has been confirmed that chronic infection of gastric mucosa with H. pylori can also induce significant increase of serum gastrin concentration9,12,14,16,26). Considering these findings, it is possible that the hypergastrinemia induced by H. pylori exerts a trophic effect on the pepsinogen-producing cells with resulting hyperpepsinogenemia I.
On the basis of our observations, we have confirmed that chronic antral H. pylori infection causes increased release of serum PG I and eradication of the organism results in significant lowering of serum PG I concentrations in patients with peptic ulcer diseases.
We are further investigating the possible causes that might be responsible for the H. pylori-induced hyperpepsinogenemia I by evaluating histologic changes of the gastric chief cells and mucous neck cells along with the degree of inflammation of the gastric mucosa.