Cisapride in Chronic Idiopathic Constipation: Clinical Response and Effect on Colonic Transit Time
Article information
Abstract
Objectives
To evaluate the effect of cisapride on the total and segmental colonic transit times (CTT’s) and on the clinical symptoms in patients with chronic idiopathic constipation, and to elucidate whether the correction of the CTT parallels the symptomatic improvement.
Methods
An open prospective trial of cisapride, 10 mg t.i.d. orally for 8 weeks, was done in 25 adult patients (M: F 6: 19) with chronic idiopathic constipation of 1 year or longer duration. CTT was measured at baseline and after 8 weeks of cisapride therapy. Frequency, consistency and difficulty in passage of bowel movements were evaluated at baseline and after 2, 4 and 8 weeks of cisapride therapy.
Results
19 cases continued to participate until the end of 4 weeks of therapy and 15 cases completed the entire 8 weeks’ trial. No serious side effect was experienced during the study period. Total and right segmental CTT’s shortened with 8 weeks of cisapride treatment. Defecation frequency increased and difficulty in stool passage improved with 2 to 8 weeks of treatment. Stool consistency had a tendency toward normal after 8 weeks of treatment (p=0.06). The global response was excellent in 7 cases (46.7%), good in 5 (33.3%) and poor in 3 (20.0%). If all dropped-out cases were assumed to be poor responders, the good or excellent responders after 8 weeks of cisapride might be 48.0% of all recruited patients. The response to cisapride could not be predicted by the various clinical parameters of the patients.
Conclusion
Cisapride is an effective drug in at least half of the patients with chronic idiopathic constipation.
INTRODUCTION
Chronic constipation is a common complaint in clinical medicine. Although dietary fiber and/or saline laxatives are often a good way of managing the symptom, some patients do not respond to this regimen and complain of more discomfort due to abdominal bloating or distension.
Korean patients with constipation usually take anthraquinone laxatives, such as senna or aloe, as a folk regimen to relieve their symptoms, and melanosis coli is quite frequently seen in such patients on sigmoidoscopy or colonoscopy. Although melanosis coli itself is not a serious problem, and is thought to be a reversible process, it may suggest a concomitant irreversible damage to the enteric nervous system1–3).
An effective and safe drug is, of course, most ideal and desirable in any disease, especially in chronic conditions lasting for years, such as constipation. cisapride is now widely used in the medical practice to promote the gastrointestinal motility, and it has been proved to be safe without serious side effects4). Cisapride may be theoretically effective for patients with constipation with prolonged colonic transit time (CTT). Several studies have shown that cisapride can improve chronic constipation5–8), but its effect in respect to the status of the CTT has not been specifically mentioned.
In patients with chronic idiopathic constipation, an open prospective trial of cisapride was done to evaluate the effect of this drug on the CTT and clinical symptoms, and to elucidate whether the correction of the CTT parallels the symptomatic improvement.
MATERIALS AND METHODS
1. Materials
Adult patients with chronic constipation for 1 year or longer duration were recruited. Each patient should have an average defecation frequency of 3 or less per week for the baseline period of at least 4 weeks. More than 50% of the bowel movements were hard in consistency and passed with difficulty. Patients with organic colorectal diseases, with endocrine diseases, in bedridden state, in pregnancy, with other serious diseases, or taking drugs that might cause constipation, were excluded. Patients who did not complete the 4 weeks of drug period for any reason were also excluded.
2. Methods
In patients with chronic constipation and in control subjects without any problem in defecation, CTT was measured using 6 gelatin capsules, each containing 10 radiopaque polyurethane markers (P. & A. Mauch, Swiss). Markers in the first and the last capsules were identical in shape, and the markers in the remaining 4 capsules were different in shape from those in other capsules. One capsule was swallowed each morning for 6 consecutive days and plain abdominal X-ray film was taken on the seventh morning. No laxative or enema was allowed during the days of measuring the CTT. Total and segmental CTT’s were calculated as previously described9–11). Colonic inertia was defined if the right segmental CTT was prolonged, hindgut dysfunction if the left segmental CTT was prolonged with normal right segmental CTT, and the functional outlet obstruction if only the rectosigmoid CTT was prolonged5).
All patients kept their diary regarding the frequency, consistency (normal, hard and very hard) and difficulty in passage (easy, rather difficult and difficult) of their bowel movements from the beginning of the baseline period to the end of the study.
Patients were treated with oral cisapride 10 mg t.i.d. 30 minutes before 3 main meals for 8 weeks. Dietary habits were continued as usual. Laxative (magnesium hydroxide 500 mg/tablet) was allowed only if a constipation episode was thought to be intractable and unbearable, and the amount of laxative tablets consumed was recorded on the diary and was checked at each visit. But other drugs of potential effects on bowel habits were prohibited during the entire period. Patients visited the outpatient clinic after 2, 4 and 8 weeks of therapy. During the 8th week, another CTT was measured. Those who did not take cisapride for more than 3 consecutive days were regarded as prematurely withdrawn from the study.
A global evaluation indicating the therapeutic effect of cisapride on symptoms of defecation was made on each patient at the end of the trial. “Excellent” response was defined if the frequency became 4 or more per week, the consistency became normal and the defecation could be done without difficulty. “Good” response was defined if there was an improvement with 1 or 2 episodes of constipation in the last 3 weeks. “Poor” response was defined if there was only slight or no improvement, i.e. the patient was still constipated in 2 out of the last 3 weeks. “Deterioration” was applied if constipation became worse.
Any adverse experience during the study was instructed to be reported and was recorded on the case record form.
All data were expressed as median (interquartile range). Wilcoxon signed-rank test, Mann-Whitney U test, Kruskal-Wallis test and x2 test were done with Stat View® II program (Abacus Concepts, U.S.A.) for the statistical analysis. If a p-value was below 0.05, it was regarded as statistically significant.
RESULTS
25 patients (M: F 6:19) were initially enrolled, but 6 of them were withdrawn before the 4 weeks of drug period. The reasons for this early drop-out were not evident in 5 cases and “no effect” was the reason in one case. Data from 19 cases who continued this trial beyond 4 weeks were used for analysis to evaluate the response of 4 weeks of drug treatment. 4 of these 19 cases could not finish the trial by the end of the 8 weeks of therapy due to “no satisfactory effect” in 3 of them and due to an unknown reason in one patient. Data on clinical response and on the CTT from 15 cases were available for the analysis regarding the response of 8 weeks of drug therapy, except one patient who did not take the radiopaque markers properly and the second CTT could not be measured.
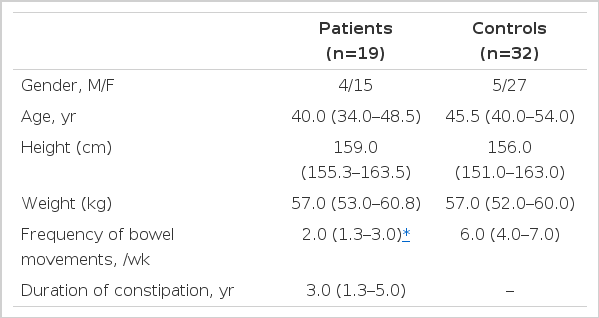
Age, gender, height and weight were not different between 19 constipated patients and 32 control subjects (Table 1). The median frequency of bowel movements was twice a week and the median duration of constipation was 3.0 years (Table 1).
No patient experienced any serious side effects during the study period. Magnesium hydroxide tablets were consumed by 2 patients, both of whom failed to continue this trial beyond 4 weeks of cisapride.
Segmental and total CTT’s in patients with constipation were significantly prolonged compared with those of control subjects (Table 2, Fig. 1). According to the initial CTT, 9 patients belonged to the colonic inertia, 5 hindgut dysfunction, 1 functional outlet obstruction and 4 normal transit time. Total and right segmental CTT’s became significantly shorter after 8 weeks of cisapride treatment, but were still longer than those of control subjects (Table 2, Fig. 1).
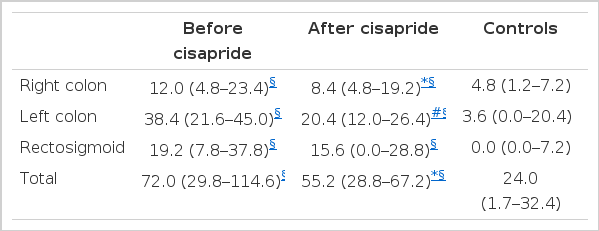
Colonic Transit Time (hrs) in Patients with Constipation Before and After 8 Weeks of Cisapride Treatment and in Control Subjects; Median (interquartile range)
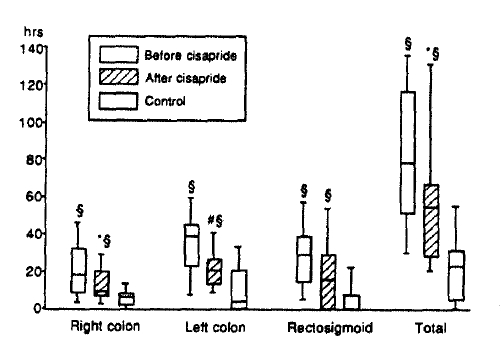
Segmental and total colonic transit times in patients with chronic constipation before and after cisapride and in control subjects. The whiskers, the margins of the box and the middle line in the box represent 90th, 75th, 50th, 25th and 10th percentiles, from top to bottom, respectively.
*pä0.05 vs. before cisapride (Wilcoxon signed-rank test)
#p=0.06 vs. before cisapride (Wilcoxon signed-rank test)
§pä0.05 vs. controls (Mann-Whitney U test)
Frequency of defecation increased significantly after 2 to 8 weeks of cisapride treatment (Fig. 2). Stool showed a tendency toward normal consistency after 8 weeks of cisapride treatment, but the statistical significance could not be attained (p = 0.06, Fig. 3). Subjective difficulty in stool passage became improved with 2 to 8 weeks of cisapride treatment (Fig. 4).
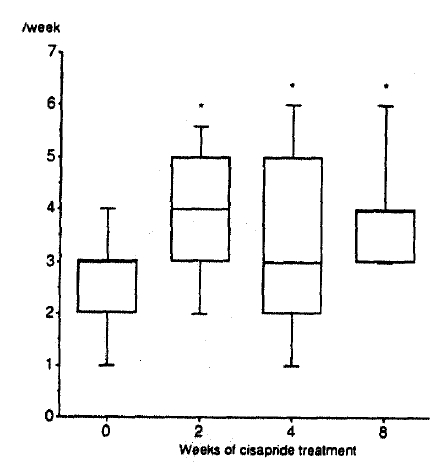
Frequency of defecation before and after cisapride. The whiskers, the margins of the box and the middle line in the box represent 90th, 75th, 50th, 25th and 10th percentiles, from top to bottom, respectively.
*pä0.05 vs. before cisapride (Wilcoxon signed-rank test)
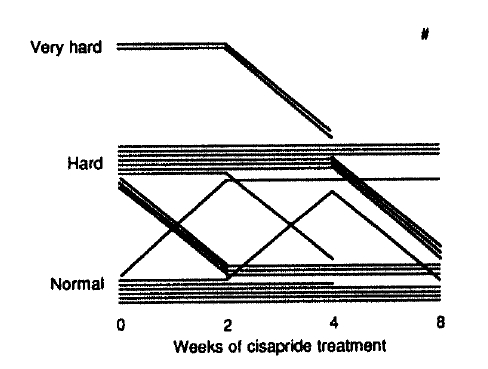
Stool consistency before and after cisapride treatment.
#p=0.06 vs. before cisapride (Wilcoxon signed-rank test)
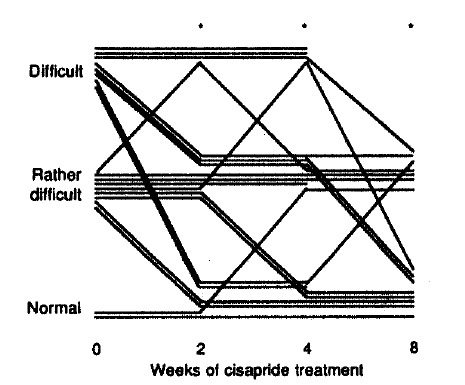
Difficulty in stool passage before and after cisapride treatment.
*pä0.05 vs. before cisapride (Wilcoxon signed-rank test)
After 8 weeks of cisapride treatment in 15 patients, the global evaluation of the response revealed as excellent in 7 cases (46.7%), good in 5 (33.3%) and poor in 3 (20.0%). If an evaluation had been made after 4 weeks of treatment in 19 patients, the excellent response would have been found in 8 cases (42.1%), good response in 5 (26.3%) and poor response in 6 (31.6%). If all dropped-out cases were assumed to be poor responders, the good or excellent responders after 8 weeks of cisapride might be 48.0% of all intially recruited patients.
The responses to cisapride were not different according to the groups of different initial baseline CTT status (i.e. colonic inertia, hindgut dysfunction, functional outlet obstruction, and normal transit time). Between excellent/good responders and poor responders, no difference was found in the various clinical parameters of the patients, such as age, gender, height, weight, duration of constipation, presence or absence of previous treatment, initial stool consistency and initial degree of difficulty in defecation. The second CTT and the difference between baseline and second CTT’s were also not different between good/excellent responders and the poor responders.
DISCUSSION
Constipation is a common complaint causing much disability in everyday life for years in those who are affected5). Increasing dietary fiber is a usually recommended way to cope with this problem. But this recipe is not always successful due to the difficulty in changing dietary habit, and sometimes due to the aggravation of abdominal fullness and bloating without remarkable effect on bowel habit. Koreans usually take plants, such as senna or aloe, containing anthraquinone alkaloids to treat constipation as a folk regimen. “Natural” plants are believed to be more harmless than the “manufactured” chemicals. But it is well known that the prolonged use of these alkaloids can cause melanosis coli and, in the long run, cathartic colon with irreversible damage to the enteric nervous system1–3,5,12). Both efficacy and safety are very important in therapeutic medicine, especially in chronic conditions lasting for years like constipation.
CTT is delayed in most patients with constipation5). Nearly 80% of our patients had delayed CTT, either totally or segmentally. Cisapride is a safe and widely prescribed drug, the main action of which is to promote the gastrointestinal motility and accelerate the CTT4,13). Therefore, cisapride is theoretically a very attractive drug for the treatment of constipation. Several trials were carried out and cisapride was proved to be an effective drug in patients with constipation5–8), but the relationship between the status of CTT and the efficacy of the drug on the constipation was not discussed clearly in these studies.
Krevsky et al. previously had demonstrated in healthy subjects the effect of oral cisapride on CTT, especially on the ascending and transverse colon14). In our patients with chronic constipation, total and right segmental CTT shortened after 8 weeks of cisapride treatment, and the efficacy of cisapride on colonic transit time, especially over the proximal colon, was proved. CTT of our patients shortened after cisapride but still not to the level of the control subjects. It is not clear whether long-term use of cisapride for more than 8 weeks may improve the CTT further to the normal level.
A few studies had previously demonstrated that the number of bowel movements increased after cisapride in healthy subjects and in constipated patients6,8,15). Frequency of defecation increased in our patients after 2 weeks of cisapride and during the whole study period. But the effect may not be maintained after cessation of the drug according to a recent study8), where the therapeutic effect of cisapride gradually disappeared with tapering the dose below 15–20 mg/day. Subjective difficulty in stool passage was also improved after 2 weeks of cisapride treatment in our patients and the effect persisted until the end of the study. But the effect on stool consistency was not evident after cisapride, although the p-value was approaching to the significance level by the end of 8 weeks of therapy. And a conclusion cannot be made firmly regarding whether or not the stool consistency would be improved significantly if the treatment is extended longer.
The global evaluation after 8 weeks of cisapride treatment was excellent or good in 80% of the completed cases. But assuming all drop-out cases were poor responders to avoid the selection attrition effect, the good or excellent responders might be about a half of the patients after 8 weeks of therapy. The response was not different according to the initial CTT status or other various clinical parameters. The global response did not parallel with the degree of effect on the CTT.
In conclusion, oral cisapride, 30 mg/day, has an effect to shorten the CTT and has beneficial effects on the frequency and subjective difficulty in the passage of bowel movements. Cisapride is an effective drug in at least half of the patients with chronic idiopathic constipation. This drug may be useful in constipated patients in whom the ordinary dietary manipulatons fail or dysmotility-like symptoms are combined.