Extrahepatic Bile Duct Hepatocellular Carcinoma without Primary Hepatic Parenchymal Lesions:
— A Case Report —
Article information
Abstract
Obstructive jaundice is rarely a presenting symptom of hepatocelluar carcinoma (HCC). Most of the cases in the literature describing obstructive jaundice by HCC have a major hepatic component. Extrahepatic HCCs without primary hepatic parenchymal lesions are extremely rare.
We encountered a case of extrahepatic HCC without primary hepatic parenchymal lesions in a 36-year-old man who presented with jaundice. We extensively sought primary hepatic parenchmal lesions preperatively and postoperatively with hepatic angiography and combined computed tomography (CT) studies, such as CT arterioportography and lipiodol-CT. The patient has been followed up for 1 year without definite evidence of recurrence. We herein report an unusual manifestation of HCC.
INTRODUCTION
Obstructive jaundice is rarely a presenting symptom of hepatocelluar carcinoma (HCC)1–2). Mechanisms of HCC-induced biliary obstruction include direct ivnasion of the intrahepatic biliary system by a tumor3–4), tumor compression of the biliary radicles5), metastatic lymph node compression, pedunculated tumor extension6), tumor fragmentation or hemobilia with clot formation4,7–12) and intrabiliary tumor growth2,12–16).
Most of the cases in the literature describing obstructive jaundice by HCC have a major hepatic component. Extrahepatic HCCs without primary hepatic parenchymal lesions are extremely rare13–15). Furthermore, some of the reports seem to lack sufficient evidence that parenchymal HCC was really absent.
We experienced a case of extrahepatic HCC without primary hepatic parenchymal lesions in a 36-year-old man who presented with jaundice. We extensively sought primary hepatic parenchymal lesions preoperatively and postoperatively with hepatic angiography and combined computed tomography (CT) studieds, such as CT arterioportography and lipiodol-CT to no avail. The patient has been followed up for 1 year without definite evidence of recurrence. We herein report an unusual manifestation of HCC and discuss the implication of this type of HCC.
CASE REPORT
A 36-year-old man was admitted to Yong Dong Severance Hospital because of a 10-day history of jaundice and epigastric discomfort. He had suffered from pulmonary tuberculosis about 15 years and 6 years before. On admission, he had pruritus, icteric sclerae and skin. The breathing sound was decreased on the right lower lung field. Direct tenderness was noted on the epigastrium, but liver and spleen were not palpable.
Abnormal laboratory findings were as follows: platelets 101,000/mm3, total bilirubin 19.6 mg/dl, direct bilirubin 13.4 mg/dl, alkaline phosphatase 188 IU/L, γ-GTP 133 IU/L, α-fetoprotein 22.1 ng/ml, urine bilirubin ⧻, urobilinogen +/−. Serum HBsAg, anti-HBc Ab and anti-HBeAb were positive.
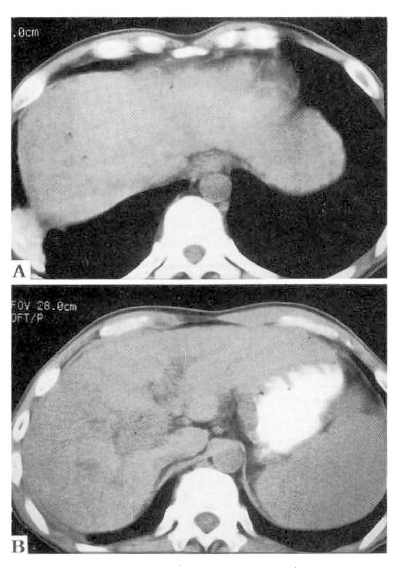
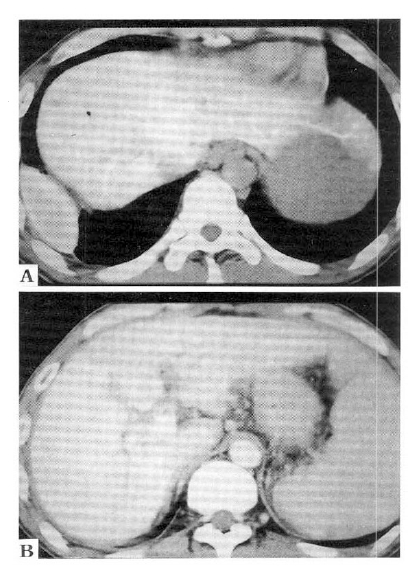
An ultrasound examination disclosed a mass at the hepatic hilus, dilatation of the intrahepatic bile duct and cirrhotic change of the liver. A dynamic abdominal CT scan showed an enhancing mass at the hepatic hilus, liver cirrhosis and splenomegaly without focal lesion in hepatic parenchyma (Fig. 1). An endoscopic retrograde cholangiography (ERC) revealed a 3×2 cm sized oval shaped filling defect at the upper common hepatic duct, including the porta hepatis. The surface of the mass was smooth and the upstream intrahepatic bile ducts were slightly dilated (Fig. 2A). An endoscopic nasobiliary drainage was attempted, but failed because a hydrophilic guidewire could not be passed over the obstructing mass. On a percutaneous transhepatic cholangiography (PTC) (Fig. 2B), through a percutaneous transhepatic biliary drainage (PTBD) tube, dye was well passed over the obstructing mass. The mass seemed slightly lobulated and the surface was shallowly grooved. A sono-guided fine needle aspiration cytology revealed no malignant cells. On the 24th hospital day, hepatic angiography was performed and did not show any tumor staining.
Dynamic abdominal C-T scan showed an enhancing mass at the hepatic hilus (arrows), liver cirrhosis and splenomegaly without focal lesion in the hepatic parenchyma.
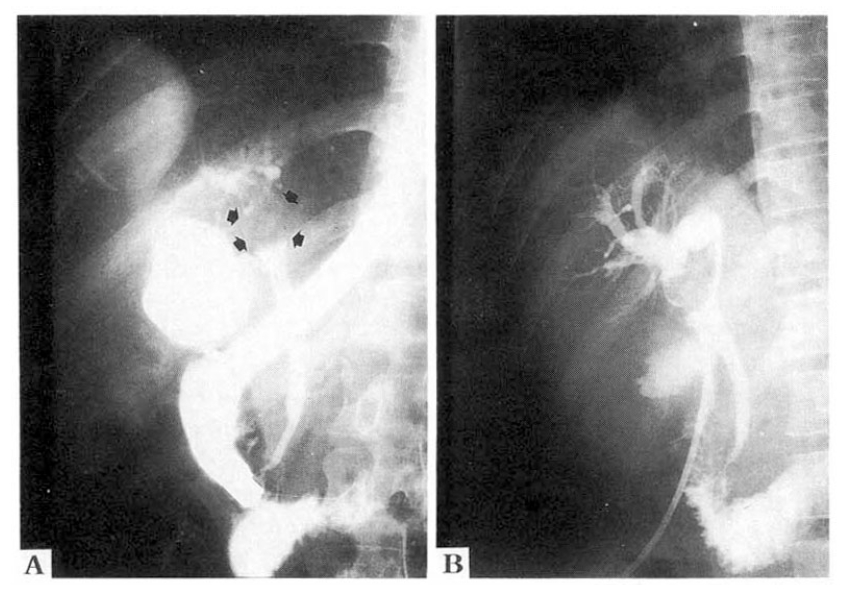
Endoscopic retrograde cholangiography (A) revealed a 3×2 cm sized, oval shaped, smooth surfaced filling defect (arrows) at the upper common hepatic duct including the porta hepatis. Note bulging of the upper common hepatic duct and the bulged and dilatation of the intrahepatic ducts. On percutaneous transhepatic cholangiography (B), the mass appeared somewhat lobulated and had shallow grooves on the surface.
On the 28th hospital day, an explorative laparotomy was performed. Grossly, the liver showed macronodular cirrhosis without any palpable masses. An irregular shaped soft tissue mass admixed with bile was found at the hepatic hilus after the distended commom hepatic duct was opened. The mass was yellowish to grayish in color and very friable. These findings were consistent with a so-called “chicken fat” appearance. Cholecystectomy, segmental resection of the intrahepatic ducts and common hepatic duct, septoplasty of the intrahepatic duct and hepaticojejunostomy were performed.
Microscopically, tumor cells appeared as nests in pseudoacinus patterns, and bile pigment was found in the cytoplasm (Fig. 3A, 3B, 3C). The immunohistochemical stain demonstrated positive reaction for α-fetoprotein but negative reaction for carinoembryonic antigen (Fig. 4A, 4B). The final diagnosis was hepatocellular carcinoma.
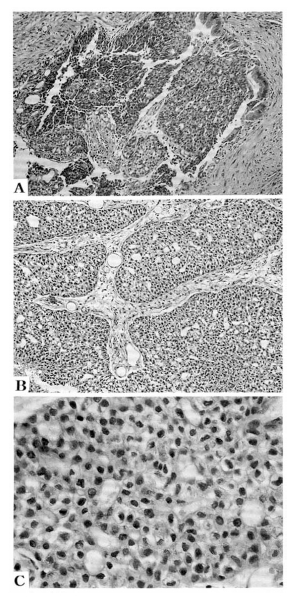
Microscopically, the tumor was located in the bile duct lumen (A, H&E, ×100). The tumor cells appeared as nests in pseudoacinus patterns (B, H&E, ×100) and bile pigment was found in the cytoplasm (C, H&E, ×400).
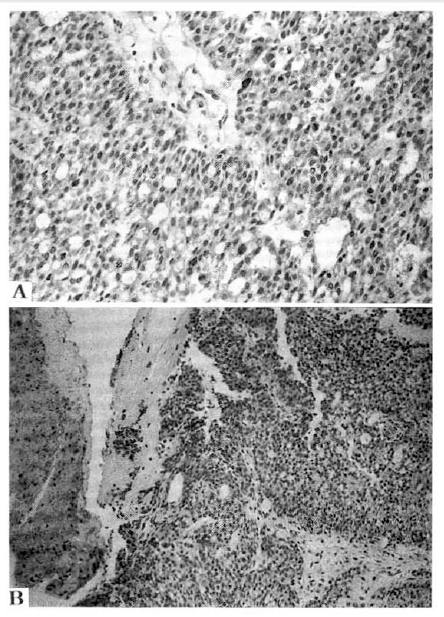
The immunohistochemical stain demonstrated positive reaction for α-fetoprotein (A, ×100) but negative reaction for carinoembryonic antigen (B, ×40).
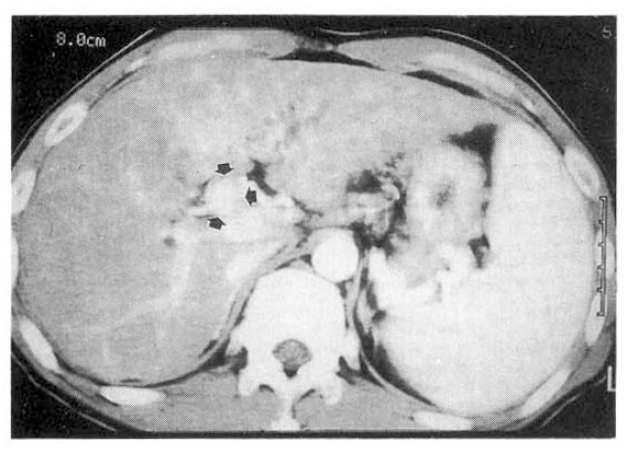
The patient was recommended to have adjuvant therapy such as external radiotherapy or systemic chemotherapy, but refused. Three months after the operation, a hepatic angiography with CT arterioportography and lipiodol-CT were performed, which gave no definite mass lesion in the hepatic parenchyma. About 6 months after the operation, a follow-up ultrasound examination disclosed two small hypoechoic lesions (1.2–1.5 cm in diameter) at the segment 8 and the segment 5 (Fig. 5A, 5B). A repeat hepatic angiography with CT arterioportography was performed. Tumor staining was not found on hepatic angiography, but two low density lesions were suspected at the segment 8 and the segment 5 on CT arterioportography (Fig. 6A, 6B). However, no lipiodol remained on lipiodol-CT taken 3 weeks later (Fig. 7A, 7B). The patient has been closely followed up with ultrasonography every 2 months until now (1 year after the operation) and there was no evidence of the growth of two hypoechoic lesions seen on ultrasonography or development of new lesions.
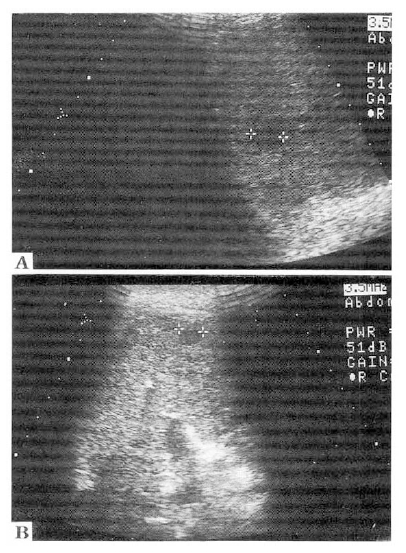
A follow-up ultrasound examination performed at postoperative 6 months disclosed two small hypoechoic lesions at the segment 8 (A) and the segment 5 (B).
CT arterioportography taken at postoperative 6 months suggested two low density lesions at the segment 8 (A) and the segment 5 (B) of the liver.
DISCUSSION
The present case is distinguished from other reports concerning extrahepatic bile duct HCC without primary hepatic parenchymal lesions. We could find a case similar to ours in the Korean literature13). That case had similar cholangiographic findings, no significant elevation of serum α-fetoprotein level, associated liver cirrhosis and no detectable primary hepatic parenchymal mass on CT and at operation. However, they did not perform hepatic angiography with or without CT arterioportography or lipiodol-CT which are known as highly sensitive methods detecting HCCs, preoperatively or postoperatively. Nevertheless, if a case survives long enough to exclude a hidden hepatic parenchymal mass, it could be considered extrahepatic bile duct HCC without primary hepatic parenchymal lesions. However, Song’s report also lacks the follow-up data. Another two Korean cases reported by Park et al.1) were asserted to be extrahepatic bile duct HCC without primary hepatic parenchymal lesions. However, they also did not perform hepatic angiography with or without combined CT studies, and saw the relatively short-term recurrence of their cases after operations (2 months and 6 months).
Badve et al.14) from India described a case of intraductal hepatocelluar carcinoma with normal liver. Their case was explored twice due to recurrence, but twice local excisions of the tumors was said to be sufficient to achieve a relatively long-term survival of the patient (more than 1 year). Although their case seems to lack sufficent radiological evidence, relatively long-term survival of the patient, despite mere local excision of the tumor, support that it was really a case of extrahepatic bile duct HCC without primary hepatic parenchymal lesions.
In this context, one of the two cases reported by Kuroyanagi et al.8) seems to meet the criteria of diagnosing extrahepatic bile duct HCC without primary hepatic parenchymal lesions. They simply removed the obstructing mass from the proximal bile duct which was confirmed as well differentiated hepatocelluar carcinoma on histologic examination. Postoperative celiac angiography did not reveal any mass staining and the patient (a 36-year-old male patient) survived 5 years 1 month after the operation, although he received intraarterial chemotherapy (mitomycin C) and postoperative external radiotherapy. They reported that jaundice in their case was caused by an obstruction due to migration of the hepatoma fragment into the extrahepatic bile duct. Presumably, a minute or small parenchymal mass near the porta hepatis might have been controlled by postoperative adjuvant therapy.
Why a close follow-up in searching for a main hepatic parenchymal lesion after operation is essential is well illustrated in a case reported by Rhoe et al.10) and one of the 6 cases reported by Park et al.12). Rhoe’s case did not show any hepatec parenchymal mass either on preoperative imaging studies or at operation. However, a follow-up abdominal CT taken one month after operation revealed parenchymal lesion at the caudate lobe of the liver. Likewise, one of the 6 cases described by Park et al.12) showed a mass in the liver parenchyma near the anastomotic site on a follow-up abdominal ultrasonography taken one month after operation.
In our case presented here, we suspected that the obstructing polypoid mass in the upper common hepatic duct on direct cholangiographic examinations would be HCC, considering the patient’s HBsAg positivity and evidence of cirrhosis on imaging studies. We, therefore, preoperatively performed hepatic angiography which failed to disclose any mass staining. On operation, no mass was found or palpated in the hepatic parenchyma. After obtaining confirmative diagnosis of HCC, we performed hepatic angiography with CT arterioportography and lipiodol-CT at postoperative 3 months and at postoperative 6 months, which did not reveal any definite parenchymal mass lesions. We think that multiple small hypoechoic lesions seen on ultrasography would be regenerative nodules rather than primary or recurrent parenchymal HCCs. These lesions have closely been followed up with ultrasonography every 2 months until now (1 year after operation) and have not shown any interval changes.
The pathogenetic mechanisms of extrahepatic bile duct HCC without primary parenchymal hepatic lesions are unknown. As shown by the case of Badve et al.14) and our present case, the possibility of primary parenchymal HCCs invading or migrating into the extrahepatic bile duct seems to be low, because mere local excisions without adjuvant therapy resulted in relatively long-term survival of the patients. We, therefore, cautiously assume that this peculiar type of HCC may primarily arise from the extrahepatic bile duct.
In conclusion, extrahepatic bile duct HCC without primary hepatic parenchymal lesions has relatively good prognosis with mere local excision and this observation suggests that HCC may primarily develop from the bile duct mucosae, which has never been mentioned in the literature.