Differences in Immunophenotyping of Mucosal Lymphocytes Between Ulcerative Colitis and Crohn’s Disease
Article information
Abstract
Objectives
Immunologic studies have characterized the numbers and types of inflammatory cells in diseased inflammatory bowel disease (IBD) mucosa but have yielded conflicting results regarding intestinal lymphocytes activation in IBD. We investigated the levels of lymphocytes subsets, interieukin-2 receptor, transferrin receptor, and T cell receptors in mainly isolated lamina propria lymphocytes, including intraepithelial lymphocytes of normal colonic mucosa or IBD (ulcerative colitis and Crohn’s disease) mucosa to understand the pathogenesis of IBD. We have results from this study.
Results
1) In comparing ulcerative colitis with control, IL-2R (p<0.05), TR (p<0.01), and CD3/HLA-DR (p<0.05) showed a significant increase.
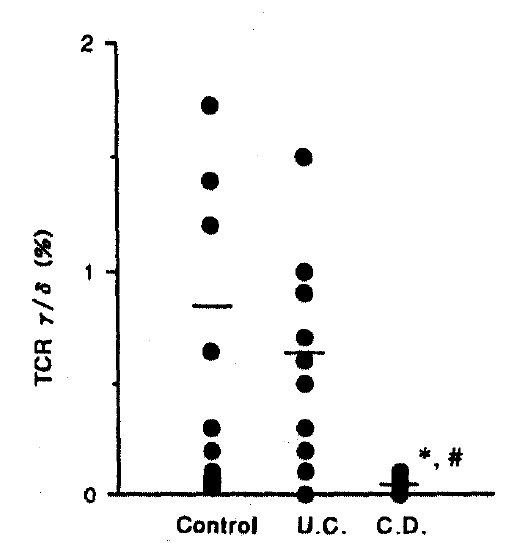
2) In comparing Crohn’s disease with control, CD3 (p<0.05), TCR α/β (p<0.01) and TCR γ/δ (p<0.05) showed a significant decrease.
3) In comparing Crohn’s disease with ulcerative colitis, CD19 (p<0.01), TR (p< 0.01), TCR α/β (p<0.01) and TCR γ/δ (p<0.05) showed a significant decrease.
Conclusion
From these results, there are increased T cell markers, IL-2R, TR, and CD3/HLA-DR in UC, but differently, decreased CD3, TCR α/β and TCR γ/δ in CD compared with control. In addition, definitive differences in lymphocytes markers, CD19, TR, TCR α/β and TCR γ/δ, which are higher in UC than in CD, may elucidate the different immunopathogenesis between UC and CD.
INTRODUCTION
The etiopathogenesis of the inflammatory bowel disease (IBD), Crohns’s disease (CD) and ulcerative colitis (UC), remains unknown. In recent years, however, considerable evidence that immunological mechanisms may play an important part in the tissue damage of CD and UC has been generated. Immunologic studies have been performed extensively in recent years to characterize the inflammatory cells in diseased IBD mucosa1,2). The histological features of IBD are consistent with increased T cell activation. CD and UC are associated with an inflammatory cell infiltate into the diseased tissue with increased numbers of T cells in the mucosa3,4). Immunotyping of T cells has failed to show any gross abnormalities in IBD. The proportions of CD4+ to CD8+ cells is similar in both diseased and normal tissue5,6) and there are no obvious defects in function7). But, the findings regarding intestinal lymphocyte activating in CD and UC are contradictory. Although a number of groups describe the increased expression of lymphocyte activation antigens including 4F2, transferrin receptor, IL-2 receptor, and HLA-DR8–10), others do not see any rise in activated lymphokines4). Flow cytometric analysis of isolated colonic lamina propria mononuclear cells demonstrated that lymphocyte activating antigens were expressed by increased percentages of intestinal B lymphocyte, CD4+ and CD8+ T lymphocytes in both UC and CD11). Isolated IBD intestinal lamina propria mononuclear cells have enhanced capacity to spontaneously release soluble IL-2 receptor, which is not specific to IBD but can also be found in colonic diverticulitis12). Native or unprimed T cells have not yet encountered processed antigen that fits their antigen receptors, and these T cells express that the surface cells will, for a short period, express high levels of the activation markers. A number of studies have demonstrated that, within the normal intestinal mucosa, a prominent population of memory (CD45RO+) T cells exists, whereas the native subset (CD45RA+) is rather small13,14). Other recent studies have demonstrated that the established CD lesion contains a significantly reduced proportion of CD45RA+ native T lymphocytes15). This preferential accumulation of memory cells has been also demonstrated by findings that the increased ratio of mononuclear cells express early activation antigens such as 4F2, primarily on B cells16). B cell proliferation and differentiation into plasma cells is dependent on cytokines and factors produced by activated T cells. Meanwhile, one of the interesting immunological findings to differentiate between CD and UC is that the mucosal CD25+ cells in CD are predominantly T cells, whereas those in UC are phenotypically macrophages.
Therefore, we designed to define the intestinal lymphocytes subsets and T cell receptors in isolated lamina propria lymphocytes, including the intraepithelial lymphocytes, derived from colonoscopic mucosal biopsy specimen from patients with IBD. We expect that this methodological approach is simple, easy to perform during colonoscopic examination, and not time-required. Moreover, we believe that the discrimination of the above immunnological parameters can help us to make a diagnosis in IBD and elucidate the differences in immunopathogenesis between CD and UC.
METHODS
1. Subjects
Fourteen IBD patients (nine ulcerative colitis, five Crohn’s disease) with a history of diarrhea, abdominal pain, abnormal findings of barium enema, colonoscopy and biopsy were selected for the study before medication. All patients, had mucosal biopsy also. Ten normal subjects also were enrolled in this study.
1) Control patients
In this group, patients had colonoscopy because of complaint of loose stool, constipation and intermittent abdominal discomfort. These patients were diagnosed as normal, irritable bowel syndrome, colonic polyp and intestinal tuberculosis. They had no evidence of inflammatory bowel disease and their colonic biopsies featured normal histology. We obtained all specimens on the colon at least 20cm apart from the lesion site.
2) Patients with inflammatory bowel disease
Biopsies of 15 patients were examined, including one relapse case of ulcerative colitis. They showed histological features of ulcerative colitis and Crohn’s disease.
2. Tissue Specimens
At least ten colonic mucosal specimens were obtained from the lesion sites, and grossly normal mucosa in control group at the lesion-free site, in the course of colonoscopy.
In the control group, any pieces from the lesion site obtained by means of colonoscopic biopsy were fixed in 10% buffered formalin and used for routine histology; the other six to seven pieces of specimen from normal mucosa were immediately immerged in RPMI 1640. These specimens were delivered to the laboratory for the study of lymphocytes subpopulations and TCR.
In the patient group, the same procedure was done from the only lesion sites. The above mentioned preparation was done also for the study of lymphocytes subpopulations and TCR.
3. Cell Preparation
The tissue specimens obtained by previous procedure were washed with HBSS and cut 1–2mm in size with forceps and scissors. For enzyme reaction, these small pieces were put into the enzyme reactor with 1 × Enzyme (Collagenase 0.02%, DNAse 40 μg/ml, Pronase 0.05%) 10ml and stored for 30minutes at 37°C in a water bath. After filtration with 25μm nylon mesh, these reactants centrifuged for 15minutes at 1500rpm. Second centrifuge was done with the resulting precipitates and HBSS for 15minutes at 1500rpm. 10% FBS/RPMI 1640 was added up to 5×105 cell/ml solution.
4. Monoclonal Antibodies (MoAbs)
Eight fluorescein isothianate (FITC) or phycoerythrin (PE) labelled MoAbs, CD3, CD19, CD45RO, and CD3/HLA-DR for the lymphocytes subpopulation, TCR δ 1, which directed against the δ chain of the T cell receptor (TCR) γ/δ, βF1, MoAb to the β chain of the TCR α/β for the T cell receptor (TCR) and MoAbs for TR (transferrin receptor) and IL2-R (interleukin 2 receptor) were purchased from Beckton-Dickinson (Mountain view, CA).
5. Stain
Each 20μl monoclonal antibody was put into each tube in the dark. 100μl of cell suspensions were added to the above monoclonal antibody contained in tubes, after shaking for 3 seconds, and incubated for 30minutes at 2–4°C. After first washing with PBS (phosphate buffer solution) 1ml, those were centrifuged for 5minutes at 1200rpm and 2–4°C. Washing and centrifuge was repeated by the above method. Finally, we obtained pellets and suspended the pellets in 1ml of PBS for assay.
6. Assay of Lymphocytes Subsets and T Cell Receptors
Cell fluorescence was analyzed with the FACScan™ Flow Cytometer (Beckton-Dickinson Mountain view, CA). Fluorochrome labelled cells were excited with the 488nm argon laser, and FITC and PE emission spectra was identified using fluorescence signals: green at 530nm, and red at 585nm. Selective bounded variables were analyzed on a FACScan flow cytometer, using FACScan Research software (Becton-Dickinson) for data acquisition and analysis.
7. Data Analysis
We performed stastical analysis on the following 8 variables: CD3, CD19, HLA-DRCD3, CD45RO, TR, T cell receptor (TCR) α/β, and TCR γ/δ. All parameters are expressed percentage to total lymphocytes count, but TCR α/β, and TCR γ/δ expressed percentage to CD3.
All factors were tested by the one group t test by the Stat view program for the Power Macintosh, model No 6100/60. All results represent mean±SE, and a P value of <0.05 is considered to be significant.
RESULTS
1. Subjects Characteristics
The mean age is 41 years old in normal, 41 years old in ulcerative colitis and 25 years old in Crohn’s disease group, and sex distribution is 4:6 (male: female) in control, 5:4 in ulcerative colitis, and 1:4 in Crohn’s disease, respectively. In inflammatory bowel disease patients, 5 subjects are Crohn’s disease, and 10 ones are ulcerative colitis, including one relapse case of ulcerative colitis. The most common chief complaint is bloody diarrhea in the patient group and loose stool without diarrhea in the normal control group. Only one patient was a relapse case, two samples were obtained at 6 months interval and the patient was managed with sulfsalazine in our out- patient department (Table 1). In the control group included 4 patients with irritable bowel syndrome, 2 patients with polyp, 2 patients with carcinoma and 2 patients with intestinal tuberculosis.
2. Lymphocyte Subsets
The percentage of CD45RO, leukocytes common antigen, shows a tendency to decrease in Crohn’s disease compared with other groups without significance, 24.00 ±4.95% in control, 23.00±5.36% in UC and 13.20±5.27% in CD, respectively.
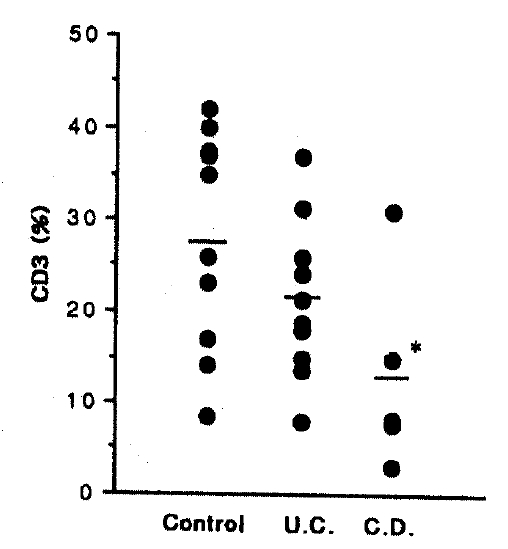
However, the percentage of CD19, 11.20±3.05% in control, 16.40±4.22% in UC and 5.60±2.29% in CD, respectively. There is a significant decrease in Crohn’s disease compared with ulcerative colitis (p<0.01), but there is a tendency to increase in ulcerative colitis and a tendency to decrease in Crohn’s disease compared with control (Fig. 1). The percentage of CD3 is 27.90±3.79% in control, 21.30±2.70% in UC, and 13.00±4.88% in CD group, respectively. This CD3 level is a significant decrease in Crohn’s disease compared with control (p<0.05), but without significance in ulcerative colitis (Fig. 2).
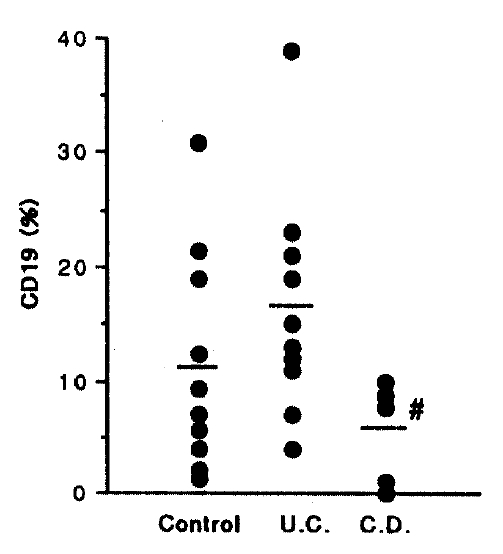
Percentage of CD19+ cells of the intestinal mucosal lymphocyte from mucosal biopsies. Significantly lesser infiltration in CD than in UC (p<0.01).
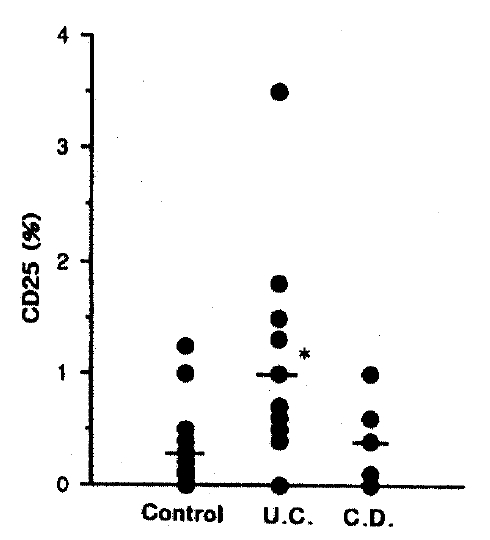
3. Interleukin 2 Receptor (IL-2R)
Fig. 3 shows the result of the percentage of IL-2R. IL-2R level in ulcerative colitis is significantly elevated (p<0.05), but no change in Crohn’s disease, 0.30±0.21% in control, 1.00±0.89% in UC, 0.40±040% in CD group, respectively.
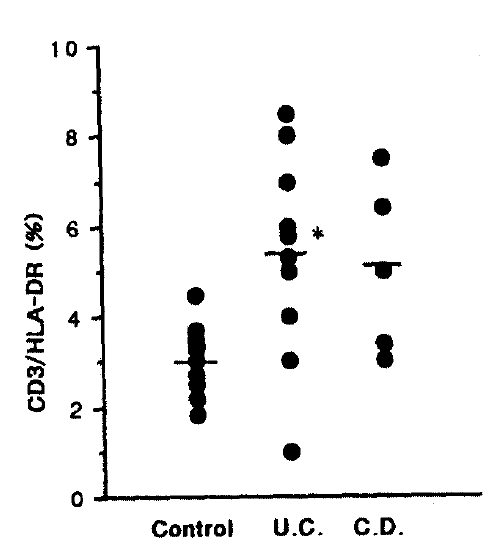
4. CD3/HLA-DR
The percentage of CD3/HLA-DR level shows a significant elevation in UC (p<0.05) and similarily in Crohn’s disease without significance, 3.00±0.57% in control, 5.33±0.85% in UC, 5.20±2.72% in CD, respectively. Fig. 4 shows the result of significant change of TR level.
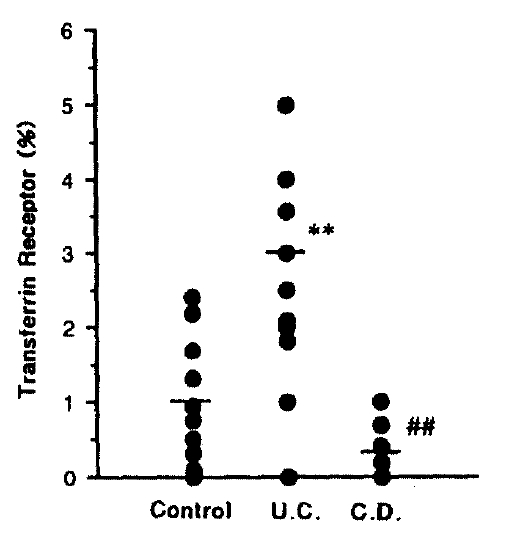
5. Transferrin Receptor (TR)
The percentage of TR shows a significant increase in UC (p<0.01) and significant decrease in Crohn’s disease compared with ulcerative colitis (p<0.01), 1.00±0.33% in control, 3.00±2.40% in UC, 0.40±0.24% in CD, respectively. Fig. 5 shows the result of significant change of TR level.
6. T Cell Receptor
In human intestinal mucosa, the intestinal intraepithelial lymphocytes (IEL) have the TCR α/β with CD8+ phenotype and of the CD3+ IEL, 1–15% have the TCR γ/δ.
The percentage of TCR α/β shows a slight decrease in ulcerative colitis, but significant decrease in Crohn’s disease (p<0.01) compared with control and UC, 23.80±3.47% in control, 18.40±3.18% in UC, 7.80±1.74% in CD, respectively (Fig. 6). Also the percentage of TCR γ/δ shows a significant decrease in Crohn’s disease (p<0.01) compared with control and ulcerative colitis, but no change in UC, 0.80±0.30% in control, 0.60±0.40% in UC, 0.10±0.10% in CD, respectively (Fig. 7).
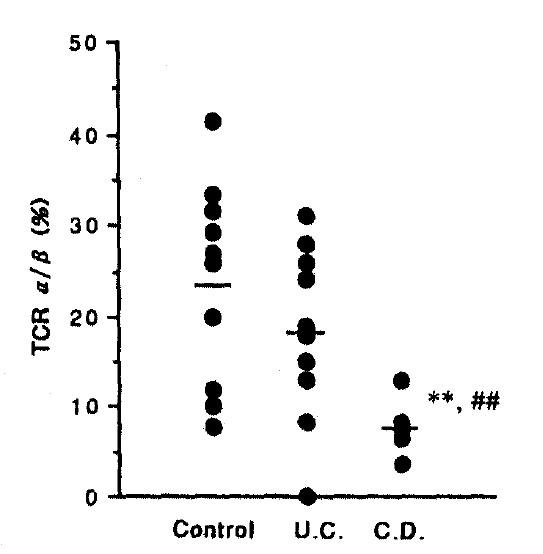
Percentage of TCR α/β+ cells of the intestinal mucosal lymphocyte from musosal biopsies. Significantly lesser infiltration in CD than in normal control (p<0.01), and in UC(p<0.01).
DISCUSSION
Lymphocytes are recirculated continuously between the blood and different tissuse of the body17). This migration ensures that the full repertoire of clonal lymphocytes specificities is available to respond to antigens throughout the body. Cytokines play a central role in modulating and regulating the immune system18). It may therefore be anticipated that they are of critical importance in maintaining or restoring mucosal immunologic homeostasis after injury. Interleukin 2 (IL-2) is a 15-kD glycoprotein produced by activated helper T lymphocytes that is responsible for activating and maintaining proliferative and cytolytic responses mediated by both T and NK (natural killer) cells19). IL-2 is essential for the proliferation, differentiation and clonal expansion of T cells and, thus, plays a central role in the regulation of cellular immunity. Normal human lamina propria mononuclear cell (LPMC) are a heightened state of activation compared with circulating lymphocytes, including increased IL-2 receptor (IL-2R) expression. An even greater proportion of LPMC expressing IL-2R has been reported in active IBD than in controls20). Increased serum IL-2 and IL-2R levels, as well as colonic tissue IL-2R levels, have been described in IBD21–23). According to our study, the percentage of IL-2R in the ulcerative colitis group was significantly elevated, and showed the tendency to increase in Crohn’s disease. From this study and result, therefore, we can suggest that local T cell activation is a feature of IBD. Furthermore, drugs that diminish IL-2 production improve the course of IBD24,25). Despite significantly elevated IL-2R level in this study, the percentage of CD4 and NK cell level saw no significant change (data not shown) and, moreover, the percentage of CD3 was significantly decreased in Crohn’s disease compared with control and there was a tendency to decrease in ulcerative colitis. These results suggest that dropped CD8 level and elevated CD3/HLA-DR level participate in an unkown mechanism which means alterations the function of lymphocytes.
Although a number of groups have described the increased expression of lymphocytes activating antigens, including 4F2, TR, IL2-R, and HLA-DR26–29), other observations have not demonstrated any rise in activated mucosal lymphocytes. The percentage of TR in our data showed significant increase in ulcerative colitis, and significant decrease in Crohn’s disease compared with ulcerative colitis. The percentage of CD19 was significantly decreased in Crohn’s disease compared with ulcerative colitis but relatively increased in ulcerative colitis compared with control and showed a tendency to decrease in Crohn’s disease compared with control, which, possibly, results from elevated IL-2R level. Taken together, these results provide evidence that alterations of B cell function and T cell lymphocytes populations occur in the intestine and some differences are likely to happen between ulcerative colitis and Crohn’s disease.
IEL contain both TCR α/β and TCR γ/δ lymphocytes, the proportions of which vary greatly with age and housing conditions (presumably a reflection of antigenic exposure). The TCR γ/δ lymphocytes, which represent the first T cells in ontogeny and display more limited diversity than TCR α/β cells30–32), are only present in much smaller numbers in the peripheral lymphoid tissues but are relatively abundant in the epithelia of epidermis, intestine, uterus and tongue33). Several studies have demonstrated that the TCR γ/δ lymphocytes are increased during infection with various pathogens, including Mycobacterium leprae34), M. tuberculosis35), M. bovis36), Leishmania donovani34), Listeria monocytogens37), Plasmodium falciparum malaria38), HIV39) and influenza A virus40). Bucht A. et al.41) reported that IBD patients demonstrated a decreased proportion of TCR γ/δ lymphocytes in the inflamed mucosa compared with the macroscopically normal area of the colon. On the other hand, a significantly increased percentage of T cells bearing the TCR γ/δ was found in peripheral blood of patients with Crohn’s disease compared with healthy individuals, including that the local mucosal inflammation may influence the circulating TCR γ/δ lymphocytes population. Also, Cuvelier et al.42) suggested that TCR α/β cells were increased amongst IEL in Crohn’s disease and TCR γ/δ lymphocytes showed no changes in IEL and LPL. In our stud, the percentage of TCR α/β shows a slight decrease in UC but a significant decrease in Crohn’s disease, compared with control and ulcerative colitis. Also the percentage of TCR γ/δ shows a significant decrease in Crohn’s disease compared with control and ulcerative colitis, but no change in ulcerative colitis. The function of TCR γ/δ, although not fully known, has been postulated as immune surveillance at epithelial surfaces and in cellular defenses against mycobacterial organisms. Our results show similar to previous reports and suggest that these decreased TCR γ/δ and functional alterations participate in pathogenesis in IBD.
We have some pitfalls in the study. The first, in that the study group in made who of few subjects, which gives rise to a bias. The second in the percentage of variances which are related to disease activity, but we negelected those because of the very small number of subjects and it was the first study in diagnosis. However, this study will contribute an understanding of the pathogenesis of inflammatory bowel disease.
In summary, our preliminary results suggest that increased expression of IL-2R, CD3/HLA-DR indicate that activated lymphocytes in inflammatory bowel disease may be able to modulate the immune response. Also, decreased TCR γ/δ and functional alterations participate in pathogenesis in IBD. However, these findings suggest that those mechanisms may be a little different between ulcerative colitis and Crohn’s disease.