Bronchioloalveolar Carcinoma in Progressive Systemic Sclerosis
Article information
Abstract
Bronchioloalveolar carcinoma(BAC) is the most common histological type of lung cancer arising in an area of scar tissue, and it is frequently superimposed in the fibrotic lung of progressive systemic sclerosis(PSS). The so called scar cancer is believed to be caused by the transformation of hyperplastic epithelium to metaplasia and finally to neoplasia under the conditions of chronic inflammation with some unknown etiological factors.
The authors report a case of BAC in a woman with the typical picture of PSS.
INTRODUCTION
Bronchioloalveolar carcinoma(BAC) accounts for less than 5 percent of all lung cancer, but it is the most common histological type associated with progressive systemic sclerosis(PSS)1). In contrast to dermatomyositis, there is no unusual frequency of other forms of malignancy in PSS.
Hale and Schatzki2) suggested a possible relationship between PSS and lung cancer initially in 1944; since then several cases have been reported3,4), and recently(1980) Talbott and Barrocas5) tabulated 44 cases. This strong relationship is now well-known and accepted and the concept of scar cancer is applied to this relationship. However, there is some skepticism concerning BAC as a complication of PSS and the basic process of this relationship is still not clarified.
CASE REPORT
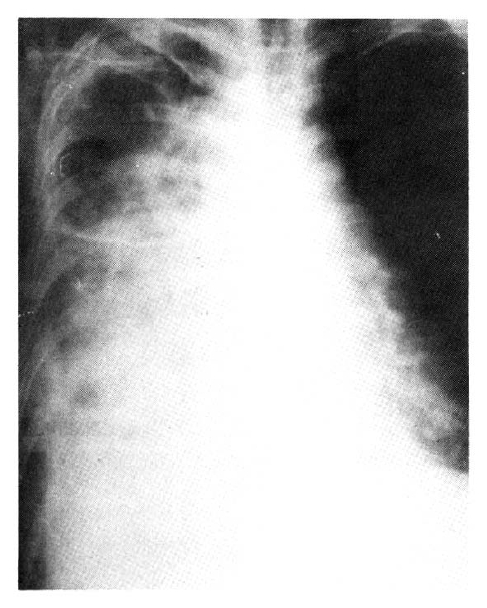
A 47-year-old housewife was admitted to our department because of dyspnea and coughing. She presented with a 20-year history of Raynaud’s phenomenon and was a non-smoker. Until the five months before admission, when she first noticed shortness of breath on exertion, she had been relatively well except for frequent upper respiratory infections. Since then her symptoms progressively worsened and seven weeks before entry she felt dyspnea on rest and exhibited a non-productive cough. She was seen at another hospital, where a pneumonia-like pulmonic infiltration in the right lower lung was noted on a chest X-ray(Fig. 1), but all microbial studies were reported to be negative.
The patient was treated with methyl-prednisolone pulse therapy under the impression of idiopathic pneumonitis, but her symptoms did not improve, hence, she was transferred to our hospital.
On admision, she looked cyanotic and tachypneic. In addition to the masked face, the skin on her hands and extremities was thickened and hypopigmented. Blood pressure was 100/70. Pulse rate was 88/min, and the respiration rate was 34/min.
Examination disclosed moderately distended neck veins, an audible rale in the whole right lung field, rapid and distant heart suounds but no murmur. The liver and spleen were not felt. In laboratory finding, CBC and urinalysis were normal. Twelve blood chemistry indices were within normal limits except total protein(5.3 g/dl) and serum albumin(2.9 g/dl).
ANA and RA factors were positive. Chest X-ray films showed an ill difined, confluent, increased density in the entire right lung field with air bronchiolo and alveologram signs(Fig. 2), and free fluid shifting on a right decubitus view. On an EKG, sinus tachycardia, low voltage, and intermittent ventricular parasystole were noted. Shortly after admission, a large amount(500 cc) of bloody exudate was drawn by thoracentesis, but such aspiration would only relieve her symptoms temporarily.
Bronchoscopic findings were a diffuse luminal narrowing with thickened mucosa. There was no endobronchial mass found, Echocardiography revealed moderate pericardial effusion and hypokinesia of the ventricles. Pulmonary function tests showed a severe restrictive pattern (FVC 1.4 L) and decreased diffusion capacity. On barium swallow, the distal esophagus was patent and atonic.
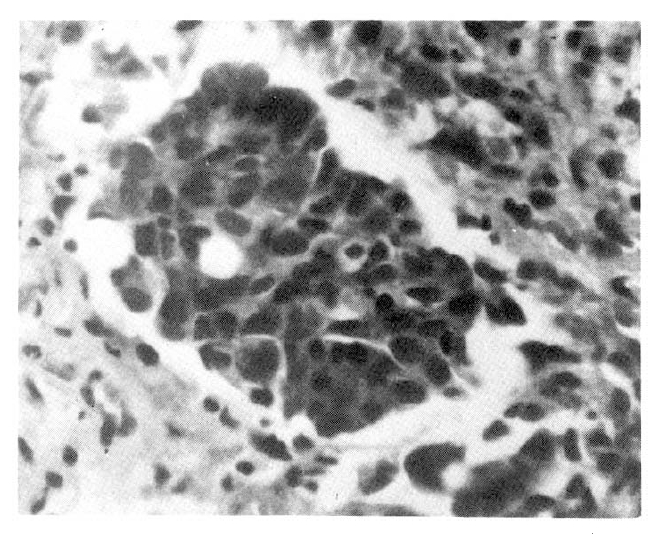
Her symptoms became more severe and the pulmonic infiltration extended, but all specimens obtained for culture at entry yielded no pathogenic organism. Thus, pleural biopsy was performed, and the authors found invading cancer cells in the pleura.
The neoplastic cells were columnar in shape and arranged linearly. Nuclei were bland and stained darkly, and with prominant nucleoli; also cytoplasm was abundant(Fig. 3).
DISCUSSION
PSS is a multi-systemic disease characterized by varying degrees of vasculitis, fibrosis, and inflammation of the skin and internal organs. Although dermatological manifestations dominate the clinical picture of this multi-systemic disease, it is the visceral organ involvement that determines survival.
The pathogenesis of scleroderma is still unknown, but microvascular change is most closely linked to it. This pathology is believed to be initiated by some cytoxic factor, not an immunologic substance like immunoglobulin, which attacks vascular endothelial cells6).
Scleroderma lung, the pulmonic involvement of PSS, is commonly found in systemic sclerosis, and it may contribute in a major way to mortality and morbidity. Interstitial fibrosis is almost always present in soleroderma lung7). Less commonly cystic lesions and pleural effusion can be noted, and pulmonary hypertension is developed in approximately 50% of PSS8) cases.
Many aspects of BAC have provoked controversy, e.g., cells of origin, biological behavior, and classification. But the outstanding feature of this neoplasm is the lepidic growth outward from its origin to line the surrounding alveolar walls which it uses both as a scaffolding and to serve as a stroma.
The incidence of BAC has been estimated at less than five percent of all lung cancers, but if peripheral lung scar adenocarcinoma were included in this group of neoplasms, as suggested by Gella and Toker9), the incidence could be good deal higher, Even without considering the WHO classification (1978), which describes BAC as a variant form of adenocarcinoma, in some cases, it is not possible to discriminate BAC from adenocarcinoma10,11).
The concept of lung cancer arising in the vicinity of pulmonary scars formed previously from tuberculosis, infarction, abscess, or interstitial fibrosis, etc., has been well established by a number of studies12). The scar cancer concept is also applied to the relationship between BAC and PSS, although the basic process is not yet clarified.
In PSS, the atypical proliferation of alveolar epithelium is noted in the vicinity of fibrotic lung tissue, Therefore, with a long standing immunological imbalance and poor removal of pollutant carcinogens via blocked lymphatic channels by the fibrotic tissue could be thought of as a trigger mechanism in neoplastic progression of metaplastic alveolar epithelia cells13).
In summary, we shoud be alert to the developement of superimposed lung cancer in scleroderma lung and a search be made for the above-mentioned tumor when respiratory symptoms worsen or change abruptly in PSS.
And even though we do not know the mechanism of this neoplastic progression, BAC in PSS is not a sure coincidence of two rare diseases, and BAC is not a surprising complication of PSS anymore.