Percutaneous Endoscopic Gastrostomy for Enteral Nutrition
Article information
Abstract
From January to October, 1986, at Wonkwang University Hospital in Iri, percutaneous endoscopic gastrostomy(PEG) was attempted in 26 patients and was successful in 24. This study was designed to review the technique and to evaluate the efficacy of PEG. The mean operation time was 22 minutes (range: 14 to 42 minutes). After feeding started, early positive nitrogen balance was achieved in all patients. All gastrostomies functioned well throughout the patient’s survival with the longest functioning at 10 month. There were no procedure-related deaths, and morbidity was lower and less severe as compared with large-bore nasogastric tube feeding. Complications included minor wound infection in two patients, stomal growth in one patient, leaks around the tube in two patients, and intraperitoneal leak in one patient. No patient developed aspiration pneumonia or required laparotomy for complications from PEG. The gastrostomy tube was easily removed endoscopically when treatment was completed. Feeding via a large-bore tube increased the risk of aspiration pneumonia (72%) and the feeding cost via a small-bore tube with elemental diet exceeded that of PEG by more than tenfold. This author’s experience with these 26 patients has led to the conclusion that PEG is safe, easy to perform, and effective means of creating feeding gastrostomy without laparotomy or general anesthesia. The authors suggest that PEG be the preferred route of alimentation in those patients who are unable to swallow for prolonged periods of time.
INTRODUCTION
The clinical dictum “when the gut works, and can be used safely, use it” still rings true and many malnourished patients can be repleted with enteral nutrition1). It is well known that the key to successful enteral nutrition is access to the gastrointestinal tract. In general, when inadequate oral intake occurs, nasoenteric tube feeding is the route of choice. However, in the case where long-term enteral nutrition is being anticipated, feeding by tube enterostomy should be considered2,3).
The Levin tube has been the most commonly used nasogastric tube for enteral nutrition. However, a number of mechanical complications have been reported with long-term use of the Levin tube. Such adverse side effects have prompted the recent development of soft, small-bore, polyurethane or silicone elastomer nasoenteric tube. The small size of these tubes obviates discomfort to a patient. Only commercial formulas are suitable for small-bore feeding tubes and, therefore, cost is a major concern. The present high cost of medical care mandates documentation of the efficacy and cost-effectiveness of nutritional supports.
Since the introduction of percutaneous endoscopic gastrostomy (PEG) by Ponsky and colleagues4) in 1980, PEG has gained wide acceptance as offers a simple and effective alternative5–10). However, despite wide spread use, there are no randomized controlled studies comparing feeding via PEG with nasoenteric tube feeding. The aim of this study was to review the technique and to evaluate the efficacy of PEG in clinical practice.
MATERIALS AND METHODS
1. Subjects
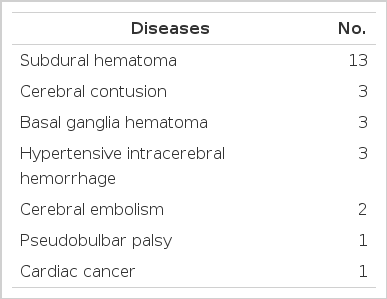
Percutaneous endoscopic gastrostomy was attempted in 26 patients ranging in age from 18 to 70 years. The patients were initially fed by nasogastric route using a No. 20 Fr. large-bore Levin tube (22 patients) or a No. 6.5 Fr. small-bore flexible Argyle tube (4 patients) for 4 to 8 weeks before the creation of PEG. Inability to swallow was the indication in 26 cases. The majority of patients (25) had severe neurologic impairments, including pseudobulbar palsy, cerebral embolism, cerebral contusion, traumatic basal ganglia hematoma, subdural hematoma, and hypertensive intracerebral hemorrhage. One patient had esophageal cancer arising from the cardia (Table 1).
2. Technique of PEG
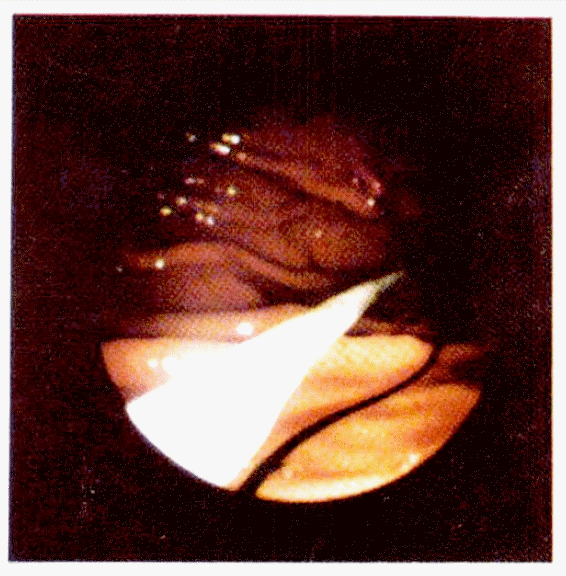
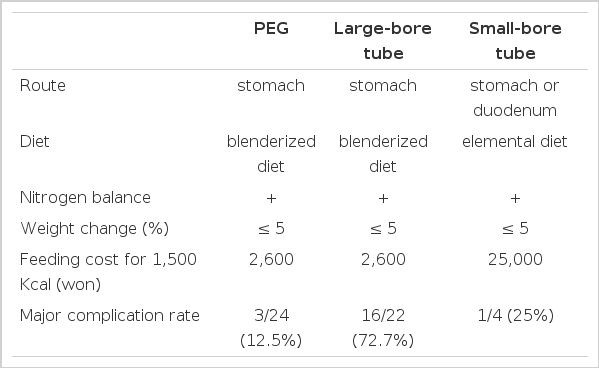
The procedure used was similar to that previously described in the literature with a few modifications4–18). Feedings were withheld for 8 hours before the procedure, and a single parenteral dose of cefamandole 1 g was given one hour prior to the procedure. Before the procedure is initiated, the gastrostomy tube was prepared by cutting off the flared end of a No. 16 Fr. Pezzer mushroom catheter and a suture was placed in the tube at this site. A tapered intravenous catheter (No. 16 Argyle medicut) was fitted to this end of the tube with a rubber seal strectched over the end. A 4 cm piece of amber drainage tubing was prepared by cutting a small opening in the side of the tubing and placing it down behind the mushroom head of the gastrostomy tube in order to provide an internal bumper (Fig. 1).
The completely assembled percutaneous endoscopic gastrostomy catheter with Internal bumper tube and dilator-like end.
A second rubber bumper was prepared from rubber drainage tubing for external use.
The patient was placed in the supine position. This position was maintained throughout the procedure and the assistant was continuously monitoring the airway and was frequently suctioning the posterior part of the pharynx to avoid aspiration. The abdominal wall was prepped with betadine and draped in a sterile fashion. Shaving was minimal and limited to the left upper quadrant. After adequate sedation with intravenous meperidine and diazepam, a pediatric gastroscope (GIF P2, Olympus, Japan) was introduced into the pharynx and it was passed distally.
The esophagus and stomach were inspected, and the stomach was insufflated with air to the point of disappearance of the stomach folds. The room lights were dimmed enabling the assistant to see where the gastroscopic light transilluminated the abdominal wall. Distinct transillumination had to be apparent at the chosen area and the authors considered this mandatory; if the abdomen cannot be transilluminated, the procedure was not attempted. As a guide for the site of tube insertion, a point was chosen on the abdominal wall near the midline and interiorly approximately one-third of the distance from the left costal arch to the umbilicus. Finger pressure applied by the assistant at this point caused a marked indentation on the interior of the stomach, which could be viewed by the endoscopist and would indicate the area at which the puncture occurred.
When both the endoscopist and assistant, have agreed on the puncture site, the skin of the chosen area was infiltrated with 2% lidocaine. A 5 mm incision was made through the skin with a No. 11 scalpel blade. The endoscopist inserted a polypectomy snare while the assistant thrusted a No. 16 Argyle medicut with syringe and needle across the adjacent abdominal and gastric walls. The endoscopist snared the catheter with the polypectomy snare and tightens the snare to hold the cannula securely (Fig. 2). The stylet was then removed from the medicut and a long (60 inches) No. 1 black silk suture was passed by the assistant through the catheter into the stomach. The snare was loosened around the catheter and allowed to slip around the silk suture; itself retightened (Fig. 3).
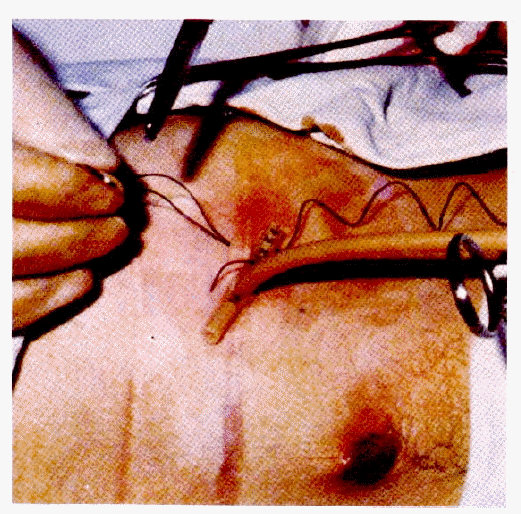
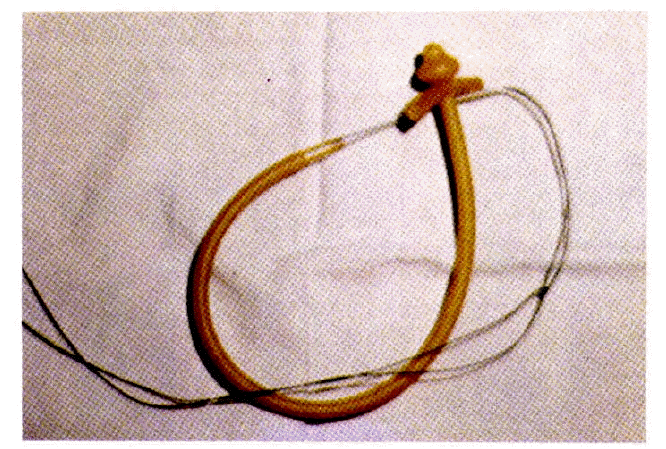
The gastroscope, snare and silk suture were subsequently removed from the patient’s mouth. The previously prepared gastrostomy tube with tapered end was tied securely to the silk suture. The gastrostomy tube was well lubricated and was held over the patient’s mouth. The assistant removed the catheter used to puncture the stomach and applies a pulling pressure on the abdominal end of the silk suture. As the pull is continued, the gastrostomy tube moved down the esophagus and into the stomach. Following the catheter’s protrusion from the abdomen, the endoscope was reinserted, and under direct observation, the endoscopist instructed the assistant to continue traction until the bumper tube (crossbar) was in contact with the gastric wall (Fig. 4).
The mushroom head of the PEG catheter and the cross-bar in the stomach. The catheter is pulled from the abdomen until the crossbar comes in contact with the gastric mucosa. Excessive tension is avoided.
Excessive tension was avoided and a second bumper tube was pulled over the tapered dilated end of the tube and fitted down to the skin (Fig. 5). Suture save placed into the skin under the bumper tube and tied to the bumper tube and to the catheter. The gastroscope was then removed after a 10 ml test dose of water was delivered and was seen entering the stomach. The dilator end of the tube was cut away and a luer lock fitting was placed over the free end of the tube. Additional doses of an intravenous cefamandole were continued for 24 to 48 hours depending on the condition of the patient.
The gastrostomy tube was removed endoscopically at a later date. The tube was cut away and the external bumper tube was removed. The gastroscope was inserted into the stomach. Under direct vision, the endoscopist snared the PEG catheter with a polypectomy snare while the assistant pushed the catheter into the stomach with a stylet. Next, the gastroscope, snare, and the PEG tube were pulled out of the patient’s mouth. No suture was placed around the remaining stoma.
3. Feeding Method and Nutritional Assessment
Feedings began the following day. After 24 hours, if bowel sounds were present and the abdomen was nontender, feedings were started with a 100 to 250 ml blenderized diet (0.5 to 1 Kcal/ml) every 4 hours. Feedings were increased, as tolerated, up to maximum 300 ml blenderized diet (1.5 Kcal/ml) every 3 or 4 hours, if needed.
Anthropometric measurements(height, weight, triceps skinfold thickness, and midupper arm circumference), biochemical testing (creatinine-height index, serum albumin, serum transferrin, nitrogen balance, total lymphocyte count), and recall skin antigen testing were done. Each objective test, measurement, and calculation was repeated serially every week or every two weeks to assess the patient’s response to feeding. Total daily caloric requirements were calculated by the Long modification19) of the Harris-Benedict equation. Protein requirements per day were calculated as follows:
Water (ml) was given to maintain water: Kcal ratio of 1:121,22), or approximately 35 ml/kg23). Water requirements were increased above normal when extrarenal fluid losses were suspected.
RESULTS
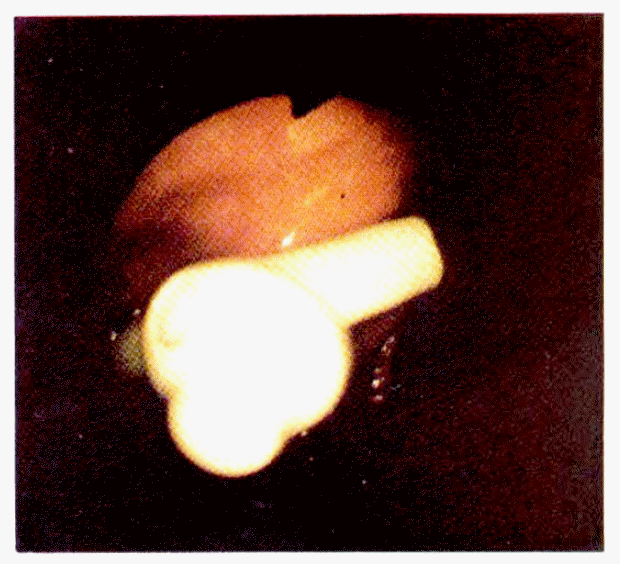
The patients were initially fed via a nasogastric tube. The candidates for PEG were referred to the Division of Gastroenterology due to frequent bouts of aspiration pneumonia or needs for long-term passive nutrition. Common indications for PEG were neurologic disorders associated with impaired swallowing, but only for those with an intact gut. PEG was attempted in 26 patients and was succesful in 24 (Table 2). In one traumatized patient with subdural hematoma and liver injury, the long silk was broken out during the steady pull on the abdominal end of the long silk suture. Another patient the authors failed had a large ventral hernia (Fig. 6) on the epigastrium and the assistant could not thrust the medicut into the stomach. All patients tolerated the procedure well and no deaths were encountered. The mean operation time was 22 minutes (range: 14 to 42 minutes). After feeding started, an early positive nitrogen balance was achieved in all patients with feeding by a nasogastric tube or PEG. All gastrostomies remained in place and functioned well throughout the patients’ survival with the longest functioning at 10 months to date.
Large ventral hernia with dirty operation scar on the epigastrium. In this patient, catheter is not inserted into the stomach.
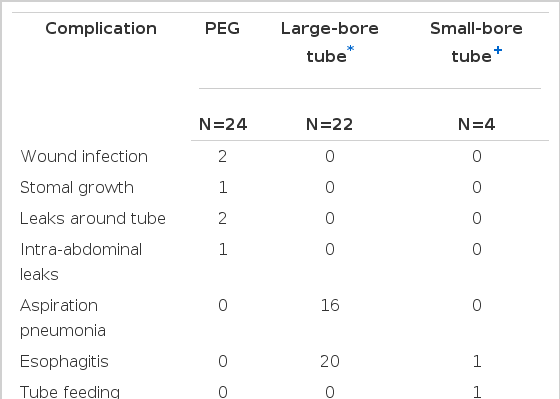
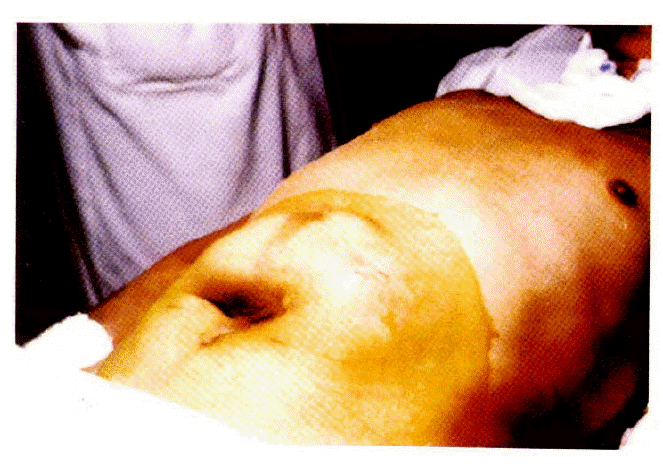
One postoperative death occurred at 74 hours, which was related to the patients underlying disease (exacerbation of intracerebral hemorrhage). Complications occurred in six patients (25%) (Table 3). Two patients developed a local wound infection around the catheter. These were easily treated by systemic antibiotics. Stomal growth around the exit site of the catheter from the abdominal wall was observed in one patient, but the catheter functioned well up eight months. Leaks around the catheter were observed in two patients. Endoscopic exploration revealed migration of mushroom head into the gastric wall due to excessive traction during the feeding (Fig. 7). These were easily treated by simply reinserting the catheter with a stylet. One patient with diabetes mellitus and subdural hematoma developed the intraperitoneal leak of feeding diet and necrosis of the exit site of catheter. These problems possibly related to excessive tension applied to the tube and to the small bumper tube. The old tube was removed by gentle traction. And this was treated by local incision drainage. The authors learned that excessive tension is undesirable.

Fresh stellate ulcer resulting from migration of PEG catheter due to excessive tension. Note the disappearance of mushroom head.
No patient developed aspiration pneumonia or required laparotomy for complications from PEG. However, with Levin tube feeding, 72% of the patient developed aspiration pneumonia and 20 had severe esophagitis. The patients(4) with elemental diet feeding via small-bore flexible tubes did not develop aspiration pneumonia. However, since only commercial formulas are suitable for small-bore feeding tubes, the daily cost was more expensive (Table 4). One patient with formula feeding by nasoduodenal tube developed the tube feeding syndrome; this was treated easily with supplemental fluids by parenteral route.
DISCUSSION
If the gastrointestinal tract is functional and accessible, it is always the preferred route for nutritional support. Nevertheless, in the seventies total parenteral nutrition (TPN) was used by many physicians and surgeons for nutritional support of many malnourished patients. In recent years with conceptual and technological advances in clinical nutrition, there has been a resurgence of interest in enteral nutrition9). The key to successful enteral nutrition is access to the gastrointestinal tract. In Korea, nasogastric tube feeding using a large-bore tube have been used most commonly. However, this technique is not well accepted due to the patient’s intolerance and high incidence of complications.
After the introduction of small-bore flexible feeding tube in Korea, many centers concentrated on enteral nutrition using a small-bore tube; however, this technique is not without complications24). Also, the comparative cost of small-bore nasoenteric tube feeding is more expensive18). Moreover, nasoenteric tubes cannot be used for long-term enteral nutrition. Hence, when prolonged enteral nutrition is anticipated, direct surgical access to the gastrointestinal tract is preferable. However, due to fear of complications, many physicians reluctant to create a surgical feeding enterostomy9).
A major breakthrough in enteral nutrition was made by Ponsky and colleagues in 1980, who developed an endoscopically placed feeding gastrostomy4). Since its introduction, PEG is being increasingly used and a number of studies suggest that PEG is a simple, relatively safe, and cost-effective means of creating enteral access for patients who require long-term passive nutrition5–10,25,26). However, despite widespread use, there are conflicting reports regarding the method and safety. Moreover, no randomized controlled trials comparing feeding by the PEG with nasoenteric tube feeding have been conducted.
Analysis of this study’s data indicate no difference on the achievement of a positive nitrogen balance with the use of each method. And problems occur mainly with the nasogastric Levin tube feeding. As a result of recurrent bouts of aspiration pneumonia via the nasogasric Levin tube feeding, the authors decided that the patients might benefit from PEG. Following PEG, there was no aspiration pneumonia noted in this study. This disparity might be due to increased LES pressure following PEG, and reduction of gastroesophageal reflux by a gastropexy-like effect of PEG27). Also, incompetance of LES by a nasogastric tube may be contributing in part to an increase in the risk of aspiration. Pulmonary aspiration can be reduced with small flexible nasoenteric tube feeding. No major complications were noted in the partients with elemental diet feeding via small-bore flexible tube. The cost, however, is a major consideration whenever elemental diet feeding is undertaken. To provide 1,500 Kcal with using a small-flexible tube may cost 25,000 Won, whereas to provide it as blenderized diet with a Levin tube or PEG costs only 2,600 Won.
Luminal obstruction occurred in three patients with nasogastric tube feeding; there was no such case with feeding by PEG. In the hospital, where the study was undertaken, the authors routinely irrigated the feeding tube with 20 to 50 ml of water before and after each feeding. Due to the longer size of nasoenteric tube it is difficult to flush them open once obstructed; however, it is easy to flush the shorter PEG tube open.
Major complications following PEG were few and not serious. The patients who developed an intraperitoneal leak were treated relatively easily. Tube displacement was noted endoscopically and it might have been the result of excessive tension applied to the tube. Presently, the authors routinely approximate the internal bumper tube loosely to gastric wall and, thus, excessive tension is avoided. The recent literature on PEG placement by various authors shows, the cumulative complication rate of 24%10). More recently published series demonstrate life-threatening complications with PEG, most notably of an infectious nature28). The worst reported complication of PEG is necrotizing fasciitis of the anterior abdominal wall which resulted in the patient’s death26,29,30). Infectious complications of PEG may be largely preventable. The value of prophylactic antibiotics for PEG has not been clarified6,30). Nevertheless, a number of authors have instituted the routine use of prophylactic antibiotics and cleansing of the oral cavity with an antibacterial solution5,9,13,18,28–35).
In conclusion, feeding via PEG or a nasoenteric tube is generally satisfactory and is an effective means for maintenance of nutritional therapy. On the other hand, large-bore tube feeding may increase the risk of pulmonary complications; however, the cost of small-bore tube feeding with elemental diet exceeds that of PEG by more than tenfold. Because of its safety, efficacy and cost effectiveness, the authors suggest that PEG be the preferred route of alimentation in those patients unable to swallow for prolonged periods of time.