Platelet Function and Factor VIII in Uremia*
Article information
Abstract
We studied platelet function and plasma factor VIII in 15 healthy individuals and 41 uremic patients to evaluate the effect of dialysis on the hemostatic defect in uremia.
Platelet counts and bleeding times were normal in all subjects. Platelet retention on glass beads was significantly reduced in all uremic patients. Platelet aggregation induced by collagen and ADP were significantly reduced in uremic patients on conservative therapy.
ADP-induced platelet aggregation was normal but collagen-induced aggregation remained abnormal in hemodialysis (HD) patients. Both ADP- and collagen-induced aggregation were normal in patients on continuous ambulatory peritoneal dialysis (CAPD). FVIII: C was normal in all uremic patients. Both FVIII:vWF and FVIII R:Ag were significantly elevated in all uremic patients.
In conclusion, platelet function was significantly defective and FVIII:vWF and FVIII R:Ag significantly elevated in uremia. HD and CAPD did not influence factor VIII levels or function nor did they improve platelet retention. Platelet aggregation improved partially on HD and completely on CAPD.
INTRODUCTION
Uremia is commonly associated with an abnormal bleeding tendency1,2). Uremic bleeding reflects a complex disorder of hemostasis and, until recently, the cause of the uremic defect in primary hemostasis has been obscure1,2). But two recent clinical studies suggested a role for factor VIII in the hemostatic defect of uremia3,4). These studies demonstrated that maneuvers that increased the level of factor VIII-von Willebrand complex in uremic patients result in a shortening of prolonged bleeding time and a transient reversal of the bleeding tendency. In uremia, however, levels of both factor VIII coagulant (FVIII:C) and factor VIII-von Willebrand factor (FVIII:vWF) have more often been found to be normal or increased3–9) than decreased10). The structure of large multimeric forms of FVIII:vWF in uremic plasma has been examined carefully and was also shown to be normal3,4,6). It is, therefore, unlikely that a specific structural or functional defect in circulating plasma FVIII:vWF could explain the hemostatic disorder in uremia.
We studied platelet function and plasma factor VIII in normal individuals and in uremic patients to evaluate the effect of dialysis on the hemostatic defects in uremia and more specifically to compare the results between hemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD). We found that FVIII:C was normal, both FVIII:vWF and factor VIII-related antigen (FVIIIR:Ag) significantly elevated and platelet retention on glass beads significantly reduced in all uremic patients. HD and CAPD did not influence factor VIII levels or function nor did they improve platelet retention. Platelet aggregation appeared to improve partially on hemodialysis and completely on CAPD.
PATIENTS
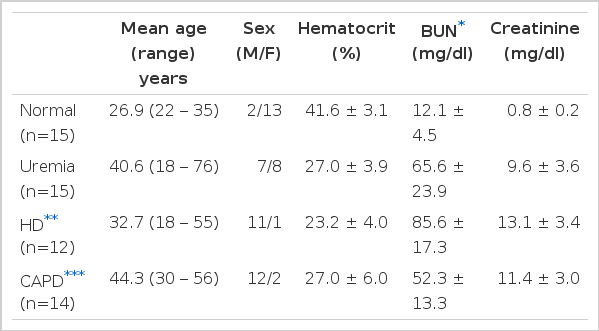
A total of 41 patients with chronic renal failure of diverse etiology were studied and the results were compared to those of 15 healthy volunteers. The clinical data are presented in Table 1. The patients with chronic renal failure were divided into 3 groups: 15 patients on conservative therapy (uremia group), 12 patients on maintenance hemodialysis (HD group: 8 hours per week on hollow fiber kidney) and 14 patients on continuous ambulatory peritoneal dialysis (CAPD group: 3 exchanges of 2,000 ml dialysate in plastic bag per day). None had taken aspirin for at least 7 days or were receiving drugs known to affect platelet function. No patients received blood transfusion during the study. Patients with known diabetes mellitus were not included in the study. In HD patients, the study was done before a dialysis and at least 48 hours after the last treatment and in CAPD patients, before an exchange of the bag.
METHODS
Bleeding times were determined by Duke method using blood lancets (Feather, Japan). Nine ml of venous blood was drawn into a plastic syringe and dispensed into a plastic tube containing 1.0 ml of 0.11M sodium citrate. Platelet rich plasma (PRP) was prepared by centrifugation of the anticoagulated blood at 150 × g for 5 min at room temperature. Platelet poor plasma (PPP) was obtained by centrifuging the remaining blood specimen at 1,500 × g for 15 min. The platelet count of PRP was adjusted to 250,000 ± 50,000 with PPP from the sample.
Platelets were counted with a Platelet Counter PL-100 (Sysmex, Japan). Platelet retention on glass beads was measured by the Salzman method11), modified using a platelet adhesiveness testing device (Igakushoinkikai, LTD, Tokyo).
Platelet aggregation studies were performed on a Bio/Data Platelet Aggregation Profiler Model PAP-3 using PAR/pak II reagents12).
The final concentrations of aggregating agents used were: epinephrine 1 × 10− 4M, collagen 0.19 mg/ml and ADP 2 × 10−5M.
FVIII:C was assayed by a one-stage method based on the partial thromboplastin time13). FVIII:vWF was measured by ristocetin-induced platelet aggregation14). FVIII R:Ag was assayed by radial immunodiffusion method based on the method of Laurell15). All values of factor VIII assay were expressed in % of normal. Two tailed unpaired t-test was used to compare the differences between groups. A p value of less than 0.05 was considered significant.
RESULTS
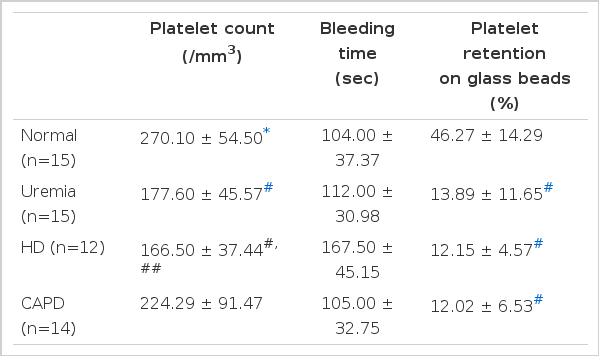
The results of platelet counts, bleeding time and platelet retention studies are shown in Table 2.
Platelet counts were within normal ranges for all patients although patients on conservative treatment and HD had counts lower than normal control. Bleeding times were normal in all patients included in this study. A tendency for longer bleeding time was noted in the HD group but the difference was not statistically significant. Platelet retention on glass beads was significantly reduced in all patients regardless of therapy. HD or CAPD did not improve defective platelet retention and there was no difference between the two dialysis groups.
The results of platelet aggregation studies are shown in Table 3. Platelet aggregation induced by epinephrine was normal in all patients. Patients on conservative therapy had significantly reduced platelet aggregation induced by collagen and ADP. In HD patients ADP-induced aggregation was normal but collagen-induced aggregation remained abnormal. In CAPD patients, platelet aggregation induced by collagen and ADP were both normal.
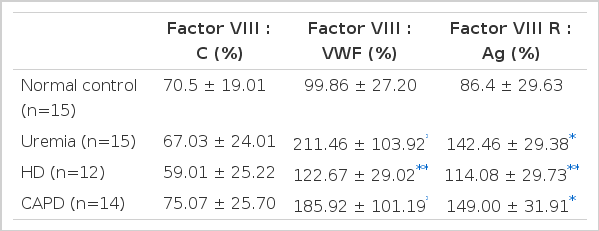
The results of factor VIII assay are shown in Table 4. FVIII:C was normal in all patients. FVIII:vWF and FVIII R:Ag were significantly elevated in all patients regardless of therapy. HD and CAPD did not influence factor VIII levels or function and there was no difference between HD and CAPD groups.
DISCUSSION
The observations that the adhesion of platelets to the subendothelial surface of rabbit aorta was strikingly decreased in patients with von Willebrand’s disease16) and that the binding of factor VIII-von Willebrand factor complex to the subendothelium of human arteries is an important step in the first phase of the hemostatic plug formation17) led investigators to evaluate the functions of the factor VIII molecule in uremia.
Factor VIII is a macromolecular complex that has two distinct biologic activities in hemostasis1,18). The first is as the cofactor required for rapid conversion of factor X to its activated form and is referred to as factor VIII coagulant (FVIII:C). The second is involved in platelet adhesion and has the ability to function as a cofactor of ristocetin-induced platelet aggregation. This activity is referred to as factor VIII-von Willebrand factor (FVIII:vWF) or factor VIII ristocetin cofactor (VIII R:RCo). The protein with this activity is referred to as factor VIII-related antigen (FVIII R: Ag).
FVIII: C appears to be a glycoprotein synthesized in the hepatocytes with a molecular weight of 250,000–300,00019). The FVIII R:Ag portion of the complex appears to be synthesized by endothelial cells and megakaryocytes and is a multimer of subunits with a molecular weight of 200,00020,21). The factor VIII complex is composed of multimeric von Willebrand factor and FVIII:C held together by noncovalent bonds.
FVIII:vWF binds to a specific platelet receptor or receptors and mediates the adhesion of platelets to subendothelial structures. FVIII:vWF circulates in a multimeric form. Its larger multimers are essential for its biologic action on platelets. Selective deficiency of larger FVIII:vWF multimers results in clinical bleeding characterized by impaired platelet adhesiveness and prolongation of the bleeding time.
In uremia, however, levels of both FVIII:C and FVIII:vWF are normal or increased as reported here and elsewhere3–9) and levels of larger multimeric forms of FVIII:vWF have also been shown to be normal4). The structure of von Willebrand multimers in uremic plasma has been examined carefully and no abnormality was found3,4,6). It is, therefore, unlikely that a specific structural or functional defect in circulating plasma FVIII:vWF could explain the hemostatic disorder in uremia.
Yet treatment that increased the concentration of all the components of the factor VIII complex shortened the bleeding time in uremic patients3,4). Thus, excessive bleeding has been successfully controlled by infusion of factor VIII concentrates (cryoprecipitate), with temporary shortening of the bleeding time3). Administration of DDAVP to patients with uremia has also prevented clinical bleeding after surgical procedures and shortened the bleeding time temporarily4). Larger von Willebrand factor multimers than those present in the resting state appeared in the plasma following DDAVP infusion and this coincided with the maximal shortening of bleeding time4).
Several possibilities are considered for these findings. It is possible that the structure and function of circulating FVIII:vWF in uremic plasma is indeed defective and the currently available assay method is not sensitive enough to detect the abnormalities. Alternatively, cryoprecipitate and DDAVP may cause shortening of the bleeding time by mechanisms unrelated to FVIII:vWF. Liu8) and Livio9) reported marked shortening of the prolonged bleeding time and control of clinical bleeding in uremic patients by administration of conjugated estrogens. Conjugated estrogens, however, did not influence the von Willebrand factor or change its multimeric structure, indicating that some other factors must be involved in the hemostatic effects of estrogens in uremia. A third possibility is that uremic platelets may lack a specific platelet-membrane glycoprotein that binds FVIII:vWF as in Bernard-Soulier syndrome22), an inherited platelet disorder with reduced platelet adhesion despite normal plasma levels of FVIII:vWF.
Although dialysis remains the mainstay of the prevention and treatment of uremic bleeding2,23,24), dialysis corrects the abnormalities only partially23,24) or only in some patients4). Nenci et al.25) reported that renal transplantation and peritoneal dialysis improved platelet aggregation while HD impaired platelet function.
Our experience showed that platelet retention on glass beads did not improve with either HD or CAPD but that platelet aggregation improved partially with HD and completely with CAPD. Our findings confirm those of previous reports23–25).
The improvement of uremic platelet abnormalities by dialysis suggests that the abnormalities are caused by retention in the body fluids of dialyzable substances which could normally be excreted by the kindey23). Indeed, phenolic acids26), guanidinosuccinic acid27), low molecular non peptidic substances28) and middle molecules29) have been found to be elevated in uremic plasma or serum and appeared to be responsible for abnormal platelet function. Some of those pletelet inhibitors may be better removed by CAPD than by HD. This is supported by the observation that CAPD offers middle molecule clearances of up to six times more per week than that with typical HD30).
The variable relationship between abnormal platelet aggregation tests and the clinical manifestations of bleeding as well as the lack of immediate correction of the prolonged bleeding time by dialysis, however, suggest a more indirect association rather than a direct causal link between the retention of uremic toxins and the hemostatic defect in uremia.
In summary, platelet retention and aggreagation were significantly reduced and FVIII:vWF and FVIII R:Ag were significantly elevated in uremia. HD and CAPD did not influence factor VIII level or function nor did they improve platelet retention. Abnormal platelet aggregation was corrected partially on HD and completely on CAPD.