A Clinical and Chromosomal Study on Those Exposed to the Atomic Bomb and their Offspring
Article information
Abstract
Presented in this paper is the data from clinical laboratory examination of 50 Korean atomic-bomb survivors (Hiroshima and Nagasaki, Japan, 1945). Of them, 15 survivors were karyotyped from their lymphocyte culture for both “stable” and “unstable” types of chromosomal aberrations. Eight of their offspring were also tested for the chromosomal changes and SCE as well.
The results are as follows:
All survivors were found to have suffered from various diseases, particularly from respiratory diseases. Two had malignancies, viz., one case having squamous cell carcinoma of uterine cervix and another, adenocarcinoma of stomach.
Serum total protein and serum albumin levels were found to have decreased in 18% and 27% of the survivors, respectively. Alkaline phosphatase, SGOT, IgG and IgM increased in 22%, 6.3%, 36.7% and 13.6% of the survivors, respectively. IgA was within normal limit in all cases.
Stable type of chromosomal aberrations was discovered in none of the survivors and the offspring examined. However, approximtely 50% of the survivors showed a significantly higher incidence of chromatid breakage than normal controls.
None of the offspring showed the chromatid breakage, but 75% of them showed significantly higher rate of SCE when compared to controls.
INTRODUCTION
Since the atomic bombing at Hiroshima and Nagasaki in 1945, the genetic effects or damages caused by A-bomb radioactivity have been reported1~3), and the first research on chromosomal aberration of A-bomb survivors was undertaken by Moorhead et al4). They first introduced the cyto-genetic method through culture of human peripheral blood.
It can be easily presumed that the damage to DNA in A-bomb surviors was due to their exposure to hundreds or thousands of atomic radioactivity of Rad.
Many Koreans who had been at Hiroshima and Nagasaki in Japan are well Known to have been damaged by the atomic bombings.
However, in korea, no investigation or study, has so far been made of genetic effects or damage by A-bomb radioactivity on Korean A-bomb survivors who returned from Hiroshima or Nagasaki.
In order to investigate the genetic effects or damage on the part of those A-bomb sufferers and their offspring for the first time in korea, we selected 50 cases at random from among 625 cases at Habchon county clinic for A-bomb sufferers in kyongsang Namdo province of Korea. Then we conducted both clinical and pathological tests for them, and applied chromosome-analysis by cytogenetic method both to 15 seriously damaged cases and 8 cases of their offspring.
SUBJECTS AND METHODS
1. Subjects
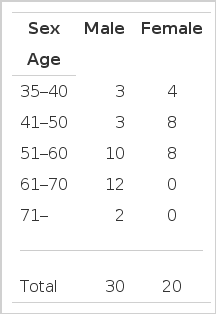
Fifty cases (30 males, 20 females) were selected at random from among 625 cases, and were given both clinical and pathological examinations. Among them, 15 seriously damaged cases were given the chromosome-analysis, and 8 offspring of the 7 cases with high frequency of chromosomal breakages were tested for chromosomal changes and sister chromatid exchange (SCE) as well.
Age and Sex distribution of the subjects is shown in Table 1. Eight cases were offspring of 5 males and of 3 females. Age ranged from ten to twenty.
2. Methods
Lymphocyte culture performed according to moorhead’4) macro-method: 10 cc of peripheral blood was taken aseptically, heparinized, and in 2 or 3 hours, separated Buffy coat was cultured for 72 hours in Eagle’s Enriched Minimal Essential (MEM) containing antibiotics, fetal bovine serum and PHA.
One or two hours before the completion of culture, Colcemid (0.1 μg/ml) was added to the cultured lymphocyte, treated with hypotonic solution (0.075 M Kcl), fixed with Carnoy’s fixative, and final chromosomal samples were made out by air-dry method.
With these samples stained by 4% of giemsa solution (Gurr’s R-66) unstable types of chromosomal aberration were examined by Seabright’s5) Giemsa banding method.
The number of observed metaphases was 30 per subject and chromosomal aberration was analysed through microscopic photography. Control subjects were examined by the same methods for chromosomal change.
In order to analyze SCE in 8 cases of their offspring, chromosomal samples were designed in the same method by adding BUdR (5-Bromodeoxy uridine, Sigma, Ultimate concentration 10−5 M) after 24 hour culture of peripheral blood and by the further 48 hour culture.
In this study quite the same brand of materials was used since fetal bovine serum (FBS-309, Gibco), culturing solution, antibiotics, Colcemid and hypotonic solution could have different effects on frequencies of SCE. BUdR addition and the proceeding thereafter were preformed in the darkroom.
Chromosomal samples were kept in the darkroom for 7 days, and then stained with Hoechst 33258 (0.5 μg/ml), exposed to UVL-56, Black Ray, Ultra-violet Products Inc., U.S., at the distance of 10 cm for 2 hours and finally stained with 4% of Giemsa for 5 to 6 minutes.
In order to analyze SCE, 14 to 18 metaphases were scored and final analysis was made by microscopic photograph (1000×) using minicopy film (ASA 25), and magnifying with Leitzphcomat II.
RESULTS
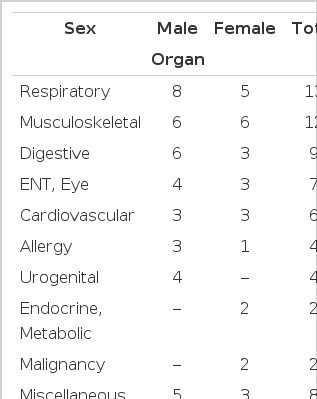
All of the A-bomb exposed patients under observation were suffering from various diseases. 2 cases were suffering from malignant tumors, Viz., 1 case from stomach cancer, 1 case, cervical cancer (Table 2).
Laboratory findings are shown in Tables 3 and 4. However, these laboratory findings seemed to have more to do with their physical illness or nutritional state than their past exposure to the A-bomb itself.
The results of chromosomal analysis on the exposed showed no unstable types of chromosmal aberrations or stable types of chromosomal aberrations.
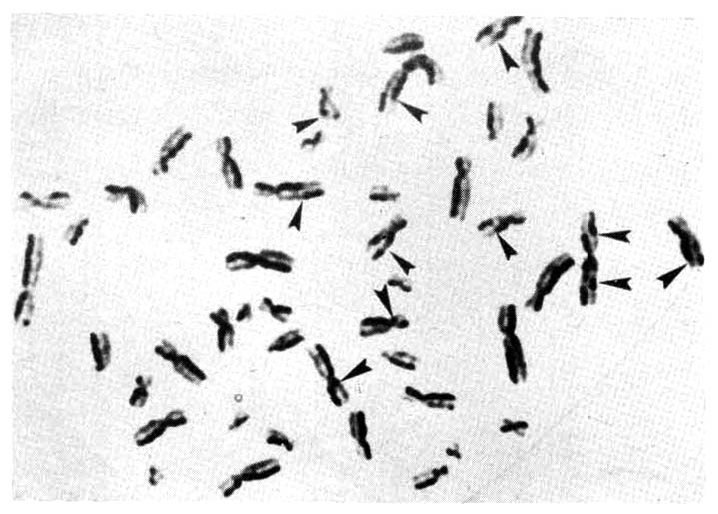
However, chromosomal breakage was observed in 7 out of 15 cases, which showed a significantly higher incidence of chromatid breakage than normal controls (Table 5, Fig. 1).
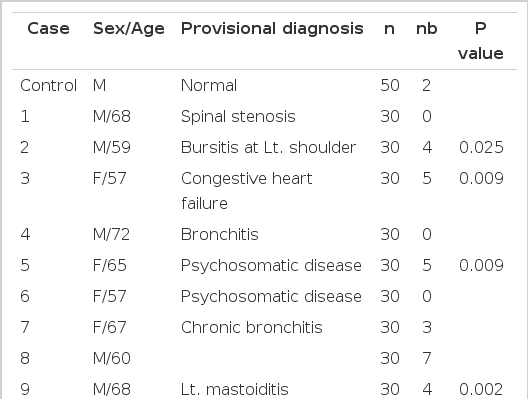
Chromosome Breakage Frequency Data for 15 A-bomb Survivors, Based on the Number of Metaphases with Breakage (s). Possible Heterogeneity between the Control and Test Subjects was Tested Via Calculation of the “Hypergeometric” Probability
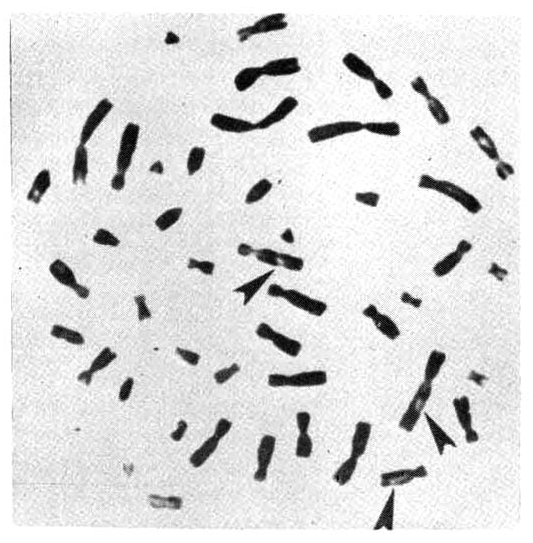
Metaphase chromosomes of a peripheral lymphocyte from an A-bomb survivor, showing chromatid breakages (←).
The results of chromosomal analysis in 8 offspring showed no structural changes in chromsomes detected, but increased frequency of SCE.
That is, 6 cases showed a significantly higher rate of SCE as compared to that of normal controls; the range of SCE frequency in 8 offspring was 3.0 to 18.0, while that of normal controls was 3.3 to 8.8 (Table 6, Fig. 2, 3).
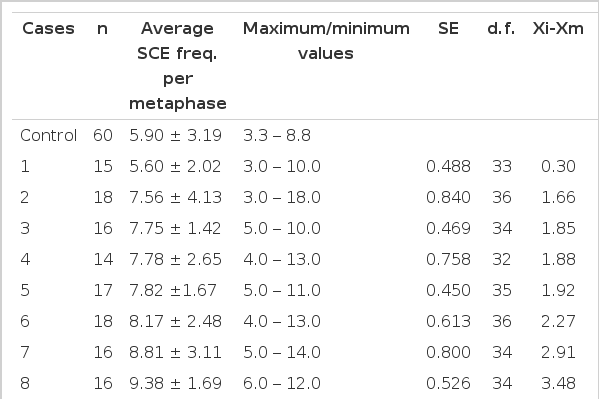
Comparison of SCE Frequencies (per metaphase) between Control and Test Subjects, with the “ANOVA” Tests of the Significance of the Difference
Lymphocyte metaphase from an offspring of an A-bomb survivor showing abnormally high numbers of SCEs (←).
DISCUSSION
Muller7) suggested for the first time, in 1927, that mutation could be made artificially to find out the vinegar-fly’s mutation caused by the exposure to X-ray. This was followed by researches by some scholars8,9) on the relation of the irradiation of radioactive rays to mutation. Moorhead et al4) introduced the cyto-genetic method by culture of human peripheral blood and it has since led many researches to the study of chromosomal aberration in the atomic bomb survivors.
The cyto-genetic method has been applied to the investigation of genetic effects or damage due to the atomic bomb radioactivity since the atomic bombings at Hiroshima and Nagasaki10~18). It is presumed that those A-bomb survivors were exposed to hundreds of radioactive rays and may be their DNA damaged.
Higher incidence of such diseases as malignant tumors, multiple myeloma and leukemia has been observed in those ever exposed to atomic radioactivity in many reports6,19,20).
This has also been identified in our study, viz., some malignant tumors were detected in 2 out of 50 A-bomb survivors.
It is assumed that damaged DNA could be responsible for genetic effects and those malignant tumors, judging from a number of studies published so far. But it has yet to be confirmed. All the cases under study were suffering from physical illness either serious or mild: 27.1% decrease in albumin and 36.7% increase in IgG of immune globulin were observed in LFT. These clinical and laboratory findings may have been due to physical damage or unfavorable nutrious conditions rather than A-bomb radiation.
Assuming that man’s exposure to the radiation would be the cause of serious health problems such as downwardness in immunity or lifespan21,22) however, it is considered necessary to make a steady and careful study of the effect by A-bomb radiation on human health.
In the analysis of chromosomes in 15 seriously affected cases, no aberrations in their number of structure were detected.
Seven out of 15 cases, however, showed a significantly higher incidence of chromatid breakage against normal controls. This higher incidence of chromatied breakage in the so-called chromosomal breakage syndrome such as Bloom’s syndrome, ataxia telangiectasia, and xeroderma pigmentosa, showed a marked decrease in chromsomal stability23).
It is somewhat hard to conclude13,24) that the higher frequency of chromatid breakage was entirely due to exposure to A-bomb radiation on the part of the survivors as many as 35 years ago.
One of the methods used in detecting damage or recovery of DNA in chromosome is the application of SCE, first used by Tayler’ et al in 195725) and then improved with the process of staining by Latt26,27) and perry et al.28,29)
This method is often used as a device for detecting genetic factors of the environmental mutation today.
The sister chromatid exchange is known to occur at S period of the cellular cycle and regarded as a mutual exchange phenomenon occurring between homologous positions on double strands of DNA.
Since the frequency of SCE is increased not only by mutagen or carcinogen, but also by clastogen of chromatid breakage19~21,30,31) the SCE could provide a parameter indicating DNA damage27,32).
The analysis of chromosomes in the survivor’s offspring whose chromatid breakage was investigated showed no aberration either in their number or structure and no difference of chromatid breakage between those subjects and normal controls. However, SCE frequency in 6 out of 8 offspring of the survivors increased in significant contrast to that of normal controls: 3.0 to 18.0 with the former against 3.3 to 8.8 with latter. This increase in SCE frequency of the offspring may not indicate aberration in chromosomal level but DNA damage in some of them, and suggests that some genetic changes would take place hereafter.