Islet Cell Surface Antibodies in Graves’ Disease; As Organ Non-Specific Antibodies
Article information
Abstract
To define ICA positiveness and its clinical correlation in AITD, ICSA were checked in Graves’ patients by indirect IF test using rat insulinoma (RINr) cells. Also Ig adherence to rat thyroid (FRTL5) and EB virus cloned human B lymphocytes that do not produce immunoglobulins were measured as the same method of ICSA with determination of organ specific antibodies in the sera.
The incidence of ICSA in Graves’ disease was 23.1 % (9/39) and the degree of the positiveness measured as % binding was roughly correlated to those of Ig adherence to FRTL5 and B cells. This ability to bind multiple organs of different species was not found to have any correlation with the titers of organ specific antibodies, but the incidence of organ specific antibody positiveness was much higher in the ICSA positive sera. Also there was a significant difference on the absorption pattern to FRTL5 and RINr cells between the sera of ICSA positive IDDM and Graves’ patients, where absorption and % binding to FRTL5, cell in ICSA positive diabetic sera were significantly lower than those to RINr cells in ICSA positive Graves’.
INTRODUCTION
Autoantibodies directed against the islet cells of the pancreas, namely ICA, have been studied mostly in insulin dependent diabetes mellitus (IDDM).1–5) ICA are usually detected in high incidence at the onset of type I DM6,7) and their prevalence is known to decrease with the increased duration of the disease.1,8) Even though it is generally regarded as a hallmark of ongoing beta cell destruction,9–14) its pathogenetic implication was blurred since it has been found in a large proportion of first degree relatives of diabetic patients.15–17) Another evidence of disease nonspecificity of ICA has been the frequent association of ICA in thyroid disease,18,19) without the nature of ICA positiveness and its significance in thyroid disease being defined.
In this paper, istet cell surface antibodies (ICSA) were checked in Graves’ sera and compared to other data to determine the characteristics and clinical correlation of ICSA positiveness in autoimmune thyroid disease (AITD), and its qualitative relationship to ICSA positiveness in IDDM.
MATERIALS AND METHODS
1. Serum Samples
Thirty nine clinically confirmed Graves’ sera (two males, thirty seven females, mean age 40 years, range 9–67 years, two new and thirty seven treated, mean duration of treatment 1.7 years, range 1 month–8 years) were obtained on a consecutive basis at the thyroid clinic after screening of diabetes by means of urine and fasting blood glucose, and anyone who showed abnormal values on the tests was excluded. Three reference ICSA positive diabetic sera (one female, 4 year of age, duration of disease less than 1 month, two males, 12 and 26 year of age, 1 and 6 years’ duration each) were obtained from the diabetic clinic and also nine healthy normal controls (all males, age range 25–34 years) who had neither diabetic and/or thyroid disease parents nor siblings were included in this study.
2. Islet Cell Surface Antibodies
ICSA were checked by the indirect immunofluorescense (IF) test using rat insulinoma (RINr) cells. Briefly, the RINr cells were grown on RPMI-1640 medium (Gibco, Chagrin falls, OH) supplemented with 10% fetal calf serum (FCS) in a humidified atmosphere of 5% CO2, 37°C and the cells were harvested with 0.05% trypsin (Difco, Detroit, MI)-EDTA in phosphate buffered saline (PBS; also containing glucose 4.0nM, tris 2%, pH 7.8). Crude immunoglobulin (Ig) fractions were precipitated from the sera after heat inactivation with polyethyleneglycol (PEG), initially mixed with 30% PEG (MW. 4000) until the final concentration of the mixture was 15% in PEG. After centrifugation at 1500 g for 60 min at 4°C, the supernatant was removed by aspiration. The assay buffer was added and recentrifuged at 1500 g for 5 min at 4°C to remove any undissolved materials. When an Ig-amount adjusted experiment is needed, IgG was prepared through protein A-sepharose CL-4B (pharmacia, uppsala, Sweden). Harvested cells (5 ×105 cells for each sample) were incubated with Ig’s (50ul each) for 30 min at 4°C, then resuspended in 50ul, fluorescein isothiocyanate (FITC) labelled anti-human IgG (Behring institute, Marburg, FGR), diluted 1:12 times in PBS and incubated for 30 min at 4°C. Before and after each step, the cells were washed three times with ice-cold PBS-2% FCS. On completion of the staining procedures, % binding was obtained on each duplicate sample through FACS IV (Becton-Dickinson, Mountain view, CA).
3. Ig Adherence to Thyrocytes and B-lymphocytes
FRTL5 cells were grown in Coon’s modified Ham’s F-12K medium (Flow lab, Irvine, Scotland) supplemented with 5% FCS and a six hormone mixture consisting of TSH (10 mlU/ml), insulin (10 ng/ml) hydroxycostisone (10−8 M), transferrin (5 μg/ml, somatostation (10 ng/ml) and glycyl-L-histidine-lysine acetate (10 ng/ml) (20). The cells were grown in a humidified atmosphere of 5% CO2 at 37°C and were transferred biweekly using a collagenase (Sigma, ST. Louis, MO)-trypsin-chicken serum mixture in calcium-magnesium-free Hank’s balanced salt solution (HBSS).
For B-cells, EB virus cloned human B-lymphocytes were used. These B-cells, derived from the peripheral blood of patient with Graves’ disease were originaly producing antimicrosomal antibody, but after a few months of subculturing, these were no longer producing any Ig and Ig was not detected by sensitive microplate radioimmunoassay (detection range 5.0-0.0 lug/ml) even on 1,000 times concentration of culture media over uncultured medium (RPMI-1640 medium supplemented with 10% FCS). Harvested cells were stained as the same method of ICSA above and % binding was obtained on each duplicate sample.
4. Absorption Studies
Harvested FRTL5 and RINr cells were reacted with Ig’s of equal volume of packed cells for 30 min at 4°C. Supernatants were obtained after centrifugation for 30 sec at 1,000g. Each sample of Ig’s unabsorbed and absorbed were then used to stain the appropriate cells as the same method above.
5. Lymphocytotoxicity
Pooled T-lymphocytes from the peripheral blood of 5 normal controls (males, aged 25–34 years) were obtained through a Ficoll-Conray gradient and sheep RBC rossette formation. Collected T-cells were then incubated with Na51CrO4 (NEN, Boston, MA), 100uCi per 1 × 106 target cells, for 1 hr at 37°C in 5% CO2 and washed three times with PBS-2% FCS. The target cells were distributed to a U-bottom 96 microwell (Linbro, McLean, VA; 5 × 104 cells/ml, 100μl/well), and incubated with sera, 100μl, diluted 1:10 times in RPMI-1640 medium for 60 min at 4°C. After centrifugation for 3 min at 500 g and aspiration of the supernatant, baby rabbit complement (Cedarlane lab, Hornby, Canada; 1:2 dilution in meida, 100μl/well) was added and reincubated for 3 hrs at 15°C with measurement of radioactivity on the supernatant thereafter.
6. Other Tests of Thyroid-Related Antibodies
a) TSH binding inhibiting antibody (TBIAb). Determination of TBIAb was carried out using solubilized porcine thyroid membranes.21,22) In brief, 50μl of solubilized TSH receptor and 50μl of receptor purified l25I-TSH were incubated for 60 min at 37°C, and after adding 350μl of assay buffer, the mixture was precipitated with 500μl of PEG and centrifuged at 1500 g for 45 min at 4°C.
The supernatant was aspirated and the radioactivity of the pellet was measured, b) Assay for antimicrosomal and antithyroglobulin antibodies. Both antibodies were checked by the hemagglutination method (Fuji Zoki, Japan) and regarded as positive when the titer was above 1 to 100. As the hemagglutination method gives only roughly graded titers, the antibodies were also determined with the microplate assay. For microplate assay, U-shaped, 96 well microplates (Falcon, Oxnard, CA) were coated with a solubilized human thyroid microsome fraction by Triton X-100 and purified thyroglobulin (Tg), 10 ug/ml each, and incubated at room temperature (RT) for 60 min. Unbound antigens were removed by aspiration and the wells were filled with PBS-1 % bovine serum albumin (BSA) for 60 min. Then, 40μl of test samples were added and incubated for another 60 min at RT. After washing the wells 5 times with PBS-1 % BSA solution, the radioactivity of each well was measured to detect the respective antibodies. Determination of the Ig amount in B-lymphocyte cultured media was also performed the same as the above procedure except the use of anti-IgG antibody instead of microsome or Tg fraction.
7. Statistics
The Bonferroni method was used for comparison of % binding on three different cell types and the chi-square test with Yate’s correction was employed for the data in Table 1 and Fig. 5.
RESULTS
1. Incidence of ICSA in Graves’ Disease
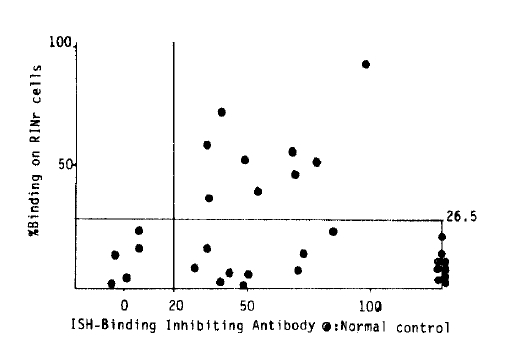
When the sera which showed % binding three standard deviations above the normal controls (26.5 %, Fig. 3), were regarded as positive, 9 cases of 39, twenty three point one percent were ICSA positive in Graves’ disease. Also to determine the effect of the amount of Ig, pooled high and low ICSA activity sera were titrated equal in amounts of IgG through the protein A-sepharose CL-4B column, but still showed high and low ICSA activity respectively (Fig. 1).
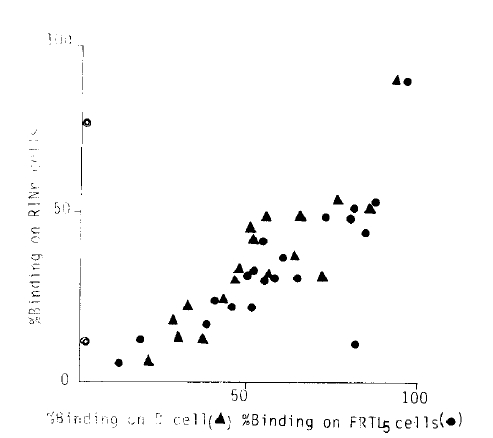
2. Correlation of % Binding on FRTL5 and B-Lymphocytes to ICSA (Fig. 1)
The individual data of positiveness measured as % binding on each of the three different cells were correlated (p>0.01, each). When the same data were rearranged to % binding on RINr and B-cells against FRTL5 cells (Fig. 2), the % binding of individual data shows FRTL5 % binding as greatest and then B and RINr cells in decreasing order (y= −10.6 + 0.87×, y = 4.9 + 0.67×; p>0.01, both).
3. Correlation of ICSA Activity to Organ Specific Antibodies
Statistically significant correlations were not found when RINr % binding was compared to the titers of each organ specific antibody including TBIAb measured by solubilized procine thyroid membranes (Fig. 3), antimicrosomal and antithyroglobulin antibodies by microplate assay and the lymphocytotoxicity test by complement mediated cytotoxicity (51 Cr release) (Fig. 4). Even though there were no correlations between individual data, when the results of % binding on RINr cells and TBIAb were combined and correlated to the data of thyroid related autoantibodies (table 1), group I, namely ICSA (+), TBIAb (+) group showed a high percentage of positiveness in antithyroglobulin and antimicorosomal antibodies, 88.9 and 100% compared to group II where 22.2 and 55.6% were positive and group III, where none was positive. Also all the patients with ICSA (+) showed TBIAb values above normal which was not true in reverse (Fig. 3).
4. Absorption Test
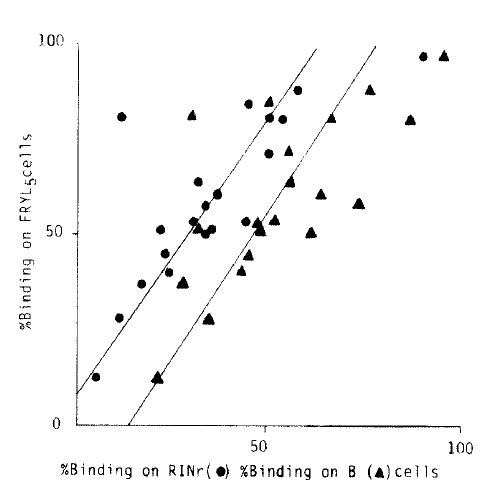
Four Graves’ and three diabetic sera which were confirmed to be ICSA positive were employed for the absorption test. Percent RINr binding and % FRTL5 binding were obtained before and after FRTL5 and RINr cell absorption. Even though the procedures of the absorption test are prone to be rough to be accurate, absorptions of Ig’s to FRTL5 cells in diabetic sera were less than 10% which were significantly different from those in Graves’ sera (mean 68%, range 47.6–91.3%, p>0.01). Also, % FRTL5 binding of diabetic sera were significantly low and within the normal range except for one case with less than one month’ duration of disease who showed % binding above normal controls (* in Fig. 5).
DISCUSSION
Antibodies that react with pancreatic islets are frequently associated with patients of newly diagnosed insulin dependent diabetes mellitus.6,7) Even though it is generally regarded as a hallmark of ongoing beta cell destruction,9–14) it also occurs in a large proportion of first degree relatives of diabetic probands15–17) and frequently in thyroid disease,18,19) making its pathogenetic implication blurred. What is perhaps not as fully appreciated is the nature of ICA positiveness and its significance in thyroid disease.
The demonstration of correlations between the individual % binding on RINr, B and FRTL5 cells strongly suggests that these antibodies in certain Graves’ sera react with cells from multiple organs of different species. Even though there could be several explanations, it seems more likely to be from the presence of multiorgan autoreactive component(s) which recognize common epitopes in different organs upon the above data and the absorption pattern where significant portion of % bindings were absorbed both by FRTL5 and RINr cells in Graves’ sera (Fig. 5). Still not clearly ruled out is the possibility that the sera of Graves’ patients contain a number of organ specific antibodies, but it is not likely to be a major portion of ICA positiveness in Graves’ patients from our data at most.
Absorption and % binding of immunoglobulins to FRTL5 cells in diabetic sera were significantly low compared to those of RINr cells in Graves’. Also noteworthy is the one case who showed FRTL5 % binding above the normal control had a duration of diabetes of less than one month. Even though it is very hard to draw any conclusion from our limited data, it seems that ICSA positiveness in type I DM is relatively more organ specific than ICSA positiveness in Graves’, supporting other data of specificities in diabetes,27,28) probably due to the difference in pathogenesis since the diabetic symptom would be the late manifestation of pancreatic beta cell destruction compared to those of Graves’, but this needs more study in the future.
The incidence of ICA in thyroid disease on the literatures varies from 2–57%,18,19) although the tests were done with several different methods of ICA determination, and in several categories of thyroid disease including thyroid nodule. Twenty three point one percent ICSA positiveness in Graves’ disease in our data is much higher than the report which showed one ICA positive case among twelve autoimmune thyroiditis patients with persistent antimicrosomal and antithyroglobulin antibodies among them new thyroiditis cases were not included.23) But the fact that all patients who showed ICSA positiveness in our data had TBIAb values above normal indicates these patients are actually in disease-active condition, besides its difference in disease composition.
Also puzzling is the mechanism of the presence of this organ-nonspecific component (s) in the active stage of Graves’ disease which is commonly regarded as an organ-specific autoimmune disease, especially the in vivo condition. It is reminiscent of experimental chronic serum sickness induced by small amounts of antigen where many kinds of nonspecific antibodies are produced initially upon the inherent ability of B cells to produce specific and nonspecific antibodies in both in vivo and vitro conditions when stimulated with nonspecific activators.24,25) The above phenomenon may possibly be explained by polyclonal stimulatiom of B lymphocytes and the diminished activity of immune suppressor system.
Our data showed no significant correlation between the ability to bind multiple organs of different species, namely ICSA positiveness. in Graves’ disease and the titers of so-called organ specific antibodies, including TBIAb, antimicrosomal and antithyroglobulin antibodies and lymphocytotoxicity. But when Graves’ patients were grouped on the presence of % binding on RINr cell and TBIAb which could be regarded as the most disease-specific among various thyroid related autoantibodies and correlated to the data of other autoantibodies merely as positive or negative, the group with ICSA (+), TBIAb (+) showed a high percentage of positiveness in antithyroglobulin and microsomal antibodies that is much higher in incidence than those of the groups without ICSA positiveness or the general incidences in Graves’ disease. The presence of the multiorgan autoreactive component (s) in AITD, therefore, could denote increased propensity to be associated with multiple organ specific antibodies. This is consisitent with the report that showed increased frequencies of autoantibodies directed against multiple organs in diabetic patients with persistent ICA positiveness26) in a certain sense, and may reflect a salubrious condition of the disease manifestation in the inherent immunologic network in certain individuals.