A Case of Subcutaneous Seeding of Hepatocellular Carcinoma After Fine Needle Aspiration Biopsy
Article information
Abstract
Cancer spread along the needle track following fine needle aspiration biopsy is said to be a rare complication. The authors report a case of subcutaneous implantation of hepatocellular carcinoma following ultrasono-guided fine needle aspiration biopsy. The patient, a 67-year-old Korean male was found to have a large hepatocellular carcinoma diagnosed by fine needle aspiration biopsy. Four months later, the patient felt two subcutaneous growing lumps at the previous aspiration site. The authors confirmed them histologically 11 months after aspiration.
INTRODUCTION
The so-called “fine” needle aspiration biopsy for cytodiagnosis of deep-seated abdominal tumors has been accepted as an inevitable diagnostic technique of the new trend since 1970, when examination of aspiration biopsy specimens was begun by Kline and Neal.1) The accurate localization and guidance of the lesion by current medical imaging methods have made it an elaborate and safe procedure. Kline and Neal describe the technique to be “essentially complication free” with an over-all accuracy of 90% .2) However, cancer recurrence along the biopsy needle track has been reported in malignant tumors of the pancreas,3,4) kidney, parotid gland, prostate, and lung.5) There have been only a few reports in cases of primary hepatocellular carcinoma since 1983, when Sakurai et al. reported the first case,6,7) We herein document another case of such an ocurrence following fine needle aspiration biopsy of a primary hepatocellular carcinoma and consider its possible implication.
CASE REPORT
A 67-year-old male was admitted to Catholic University Medical College, Kangnam Hospital on October 18, 1987, for the evaluation of a right hepatic mass that was detected incidentally by ultrasonograhy. On admission, neither jaundice nor ascites were present. The liver was about 5 cm below the right subcostal margin. The serum hepatitis B viral surface antigen and antibody were both negative. The liver function tests revealed total protein (6–8 g/dl) 7.7 g/dl, albumin (3.5–5.0 g/dl) 4.1 g/dl, globulin (2.0–3.5 g/dl) 3.7 g/dl, aspartate aminotransferase (40 units) 72 units, alanine aminotransferase (40 units) 64 units, r-glutamyl transpeptidase (upto 80 mU/ml in adult male) 294 mU/ml, and alkaline phosphatase (upto 150 IU/L) 484 IU/L. An abdominal CT scan confirmed a large ovoid inhomogeneous hypodense mass involving the right posterior area of the liver, measuring about 9×9 × 8 cm. It was inhomogeneously enhanced with well demarcation (Fig. 1). On October 20, we did a peritoneoscopy that showed neither the cirrhotic nodules nor the abnormal protruding masses within the area of the visible hepatic surface. On Oct. 23, a percutaneous ultrasono-guided needle biopsy was done by a fine needle followed by transcatheter chemoembolization, a combination of transarterial infusion of Lipiodol and mitomycin-C 2 mg. On histopathologic examination, the tissue specimens taken by peritoneoscopic guided biopsy with a Menghini needle and ultrasono-guided aspiration biopsy showed chronic persistent hepatitis (Fig. 2a) and a well differentiated trabecular hepatocellular carcinoma (Fig. 2b) respectively. The patient was discharged on November 16, 1987, with a sense of well-being.

Abdominal CT scan showed an inhomogeneously enhanced hepatic tumor with well demarcation. (9 × 9 × 8 cm).
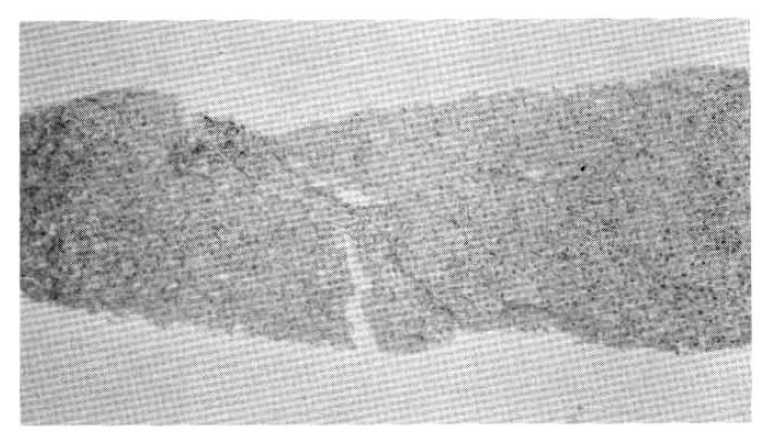
Chronic persistent hepatitis showing chronic inflammatory cell infiltration and widening of the portal tract.
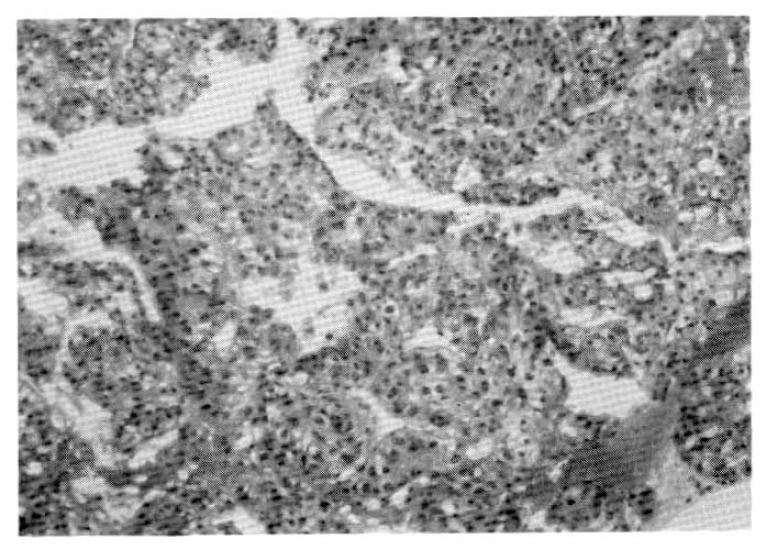
Hepatocellular carcinoma showing trabecular pattern with sinusoidal structure and pleomorphic epithelial cells resembling hepatocytes.
On June 10, 1988, he was readmitted for a regular check-up and the anticancer chemotherapy. On examination, two subcutaneous lumps were noted at the previous ultrasono-guided needle biopsy site (Fig. 3). He had first noticed them about 3 months previously, approximately 4 months after the chemoembolization. They were movable without tenderness and round in shape, measuring 2 cm in diameter. An abdominal CT scan showed a more enlarged size (10 × 10 × 11 cm) with evidence of metastasis to the abdominal wall (Fig. 4). The patient was discharged after the first anticancer chemotherapy, a combination of 5-FU, Adriamycin and mitomycin-C (FAM). The patient was followed by a second FAM chemotherapy from August 3 to 10, 1988.
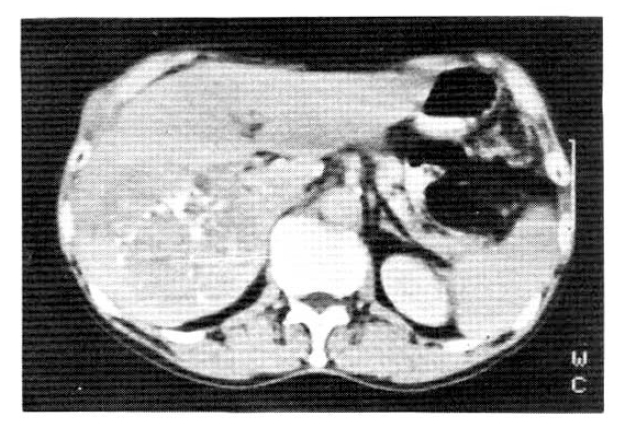
Abdominal CT scan showed a more enlarged size (10 × 10 × 11 cm) with evidence of metastasis to the abdominal wall and only a small amount of lipiodol droplets remaining in the tumor.
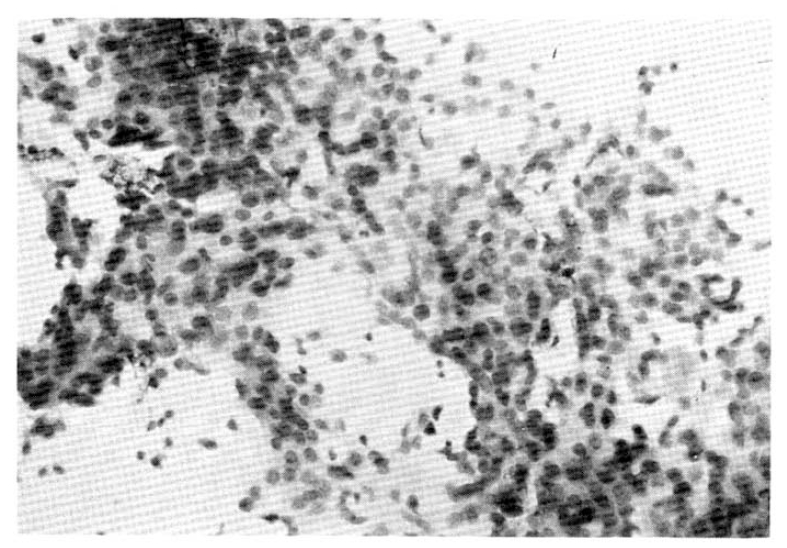
On September 9, 1988, the patient was admitted for the third FAM chemotherapy. The cancer was more enlarged in size and had made a few daughter nodules around the main lesion that were evidenced ultrasnographically. The two subcutaneous nodules were not changed in movability, size, and number. On September 15, a needle aspiration on them was done. The tumor histopathology showed an irregular sheet of malignant epithelial cells which had anisonucleosis, hyperchromatism and occasional distinct nucleoli (Fig. 5).
DISCUSSION
Hepatocellular carcinoma is one of the most common malignant tumors in Korea. The resectability rate of these cancers is very low because of late discovery of the tumor and the frequent association with cirrhosis. In recent years, however, the development of a screening program for early diagnosis of primary hepatocellular carcinoma, such as a regular check-up for alpha-fetoprotein levels and an ultrasonogram in the high risk patient group, makes it possible to discover even small tumors which can be completely cured. If a tumor is detected by the screening test or other diagnostic tools, a needle biopsy is indicated to establish an accurate diagnosis. Conventional needle biopsy of the liver has been a useful diagnostic tool for the histopathologic confirmation and prognostic assessment. But it has some serious complications including hemorrhage, bile peritonitis and injury to neighboring organs.8) Besides these, needle track seedings occur, especially in the case of a cutting needle of the Vim-Silverman type. On the other hand, the usefulness and safety of guided percutaneous fine needle aspiration biopsy of the liver have been documented as compared with conventional needle biopsy.9,10 Ho et al. recommended this technique for pathologic diagnosis of hepatic malignancy because of its simplicity, high yield, and reasonable safety.10) Some considered this procedure to be questionable because it might elicit tumor implantation in the needle track and there are a few well documented cases6,7) and experimental evidence to support such implantation.11) Therefore, guided fine needle aspiration biopsy of the liver is no longer free from cancer spread. We have rarely experiecned needle track seeding. Sakurai et al. suggested that the reason for its rarity would likely be that most primary hepatocellular carcinomas were so malignant that the ensuing life span of the patient was too short for the disseminated malignant cells to mature into detectable neoplastic masses.6)
New diagnostic developments for early detection of primary hepatocellular carcinoma will give us more opportunity to use guided fine needle aspiration biopsy for smaller cancers. Some researchers insist that needle biopsy of a malignant tumor of the liver should be performed only after all other possible diagnostic procedures fail because curative liver tumor resection can now be done with low operative morbity and mortality, and a good outcome should not be put at risk by an unnecessary percutaneous needle biopsy,7) On the contrary, others indicate that it appears unnecessary to revise the indications for hepatic needle aspiration biopsy in view of the obvious rarity of malignant implantation, as “the risk of spreading cancer by percutaneous fine needle aspiration biopsy is more of theoretical than practical significance”.12) We suggest that fine needle track implantation should not be ignored even in small hepatic cancer, especially after multiple aspirations, although it is rare. It is obligatory, however, to confirm the histologic diagnosis before the determination of suitable therapy.
Notes
A preliminary report of this work was presented at the 38th annual meeting of the Korea Internal Medicine in Seoul, Korea. October, 1986. This work was supported in part by the Catholic Medical Center, clinical research founds.