Influence of Gamma-Aminobutyric Acid on the Changes of Blood Pressure in Rats†
Article information
Abstract
This is an attempt to investigate the effect of gamma-aminobutyric acid (GABA), a well-known major inhibitory neurotransmitter in the central nervous system, on the blood pressure response in rats and to elucidate the mechanism of its action. GABA injected into a femoral vein of the rat produced a dose-related fall in blood pressure followed by a secondary pressor response.
The depressor response evoked by GABA was clearly blocked by pretreatment with chlorisondamine, diazepam and picrotoxin but was unaffected by atropine, prazosin and debrisoquin. GABA-induced pressor responses were significantly attenuated by pretreatment with prazosin or picrotoxin, while not affected by atropine, diazepam, debrisoquin and chlorisondamine.
These experimental data suggest that GABA causes biphasically depressor and pressor responses in rats, and that the hypotensive activity evoked by GABA may be exerted through activation of GABAergic receptors and hypertensive activity due to stimulation of the adrenergic alpha-receptors, which appears to be associated with GABAergic receptors.
INTRODUCTION
Gamma-aminobutyric acid (GABA) is a major inhibitory transmitter in the central nervous system1) with a wide distribution in the brain and high concentrations in the hypothalamus,2) including hypothalamic nuclei involved in cardiovascular control, such as the anterior hypothalamus or the paraventricular and supraoptic nuclei. GABAergic mechanisms control a variety of brain functions by influencing the activity of dopaminergic and serotonergic neurons.3–4)
It is known that GABA increases the secretion of catecholamines from the isolated bovine adrenal gland and the dog adrenal gland.6–7)
More recently, Lim et al.8) showed that GABA produced catecholamine secretion in isolated perfused rat adrenal gland, and this secretory effect evoked by GABA may be exerted by stimulating the release of acetylcholine through GABAergic receptors located on the chromaffin cells and/or splanchnic nerve terminals.
It has been found that administration of GABA or GABAergic agonists, such as muscimol or THIP, to the brain causes a reduction in blood pressure and heart rate in various species, including man, as has been reported by several authors.9–17) These cardiovascular actions were found to be mediated, in most instances, by specific bicuculline-or picrotoxin-sensitive GABAergic receptors.11–12,16,18) They may involve changes in central cholinergic and serotonergic activity16) and interactions with central peptidergic pressor pathways,19) as well as inhibition of the somatosympathetic reflexes of the medial medullary depressor region.20)
Recently, Unger et al.21) demonstrated that central GABAergic stimulation causes increased efferent adrenal nerve activity in conscious stroke-prone spontaneously hypertensive rats, along with a selective inhibition of sympathoadrenal pathway. They found that the antihypertensive action of central GABA receptor stimulation in stroke-prone spontaneously hypertensive rats is not mediated by an increase in vagal tone or a generalized reduction in sympathetic tone, but is associated with the selective suppression of sympathoadrenal activity.
The present study was therefore designed to investigate the intravenous actions of GABA on the blood pressure of the rat and to clarify the mechanism of its action.
MATERIALS AND METHODS
1. Experimental Animals
Mature Sprague-dawley rats of both sexes weighing 180–250 grams were used in this experiment. The animals were housed individually in cages, and food (Cheil Animal Chow) and tap water were allowed ad libitum at least for a week for their adaptation to experimental circumstances. On the day of the experiment, each rat was anesthetized with pentobarbital-Na (Nembutal-Na®) 40 mg/kg intraperitoneally. A cannulation was performed on the trachea of each rat, which was tied in a supine position to a fixing panel to prevent movement. Body temperature was maintained at 37°C–38°C with a thermostatically controlled blanket and heating lamp.
2. Determination of Blood Pressure
The right common carotid artery was catheterized with an artery cannula and connected to a pressure transducer, while the pulse pressure of the mean arterial blood pressure was recorded on a physiograph (Beckman Co.) for continuous monitoring of arterial, pressure. The artery cannula was filled with heparin solution (400 I.U.) to prevent blood coagulation during the experiment. Another cannulation with a polyethylene tube (Gauage No. 23) was made into a femoral vein for injecting drugs. Each rat was left undisturbed for at least 30 minutes after completion of the operative procedures to permit cardiovascular parameters to be stabilized.
3. Drugs
The following drugs were used in the present study: gamma-aminobutyric acid (Sigma Chemical Co.); atropine sulfate (Merk Co.); chlorisondamine chloride (CIBA Co.); prazosin hydrochloride (Pizer, Co.); debrisoquin sulfate (Korea Chongkeundang Pharmaceutical Co.); picrotoxin (Kishida Chemical Co. Japan) and diazepam injection (Korea Roche Co.) These drugs were freshly made with 0.9% sodium chloride on the day of the experiment and stored in a refrigerator for the next use. All drugs were administered into a femoral vein.
4. Statistical Analysis
The statistical significance between groups was determined utilizing the Student’s “t” test. Data obtained from animals which served as their own control were analyzed for significance using the t-test for paired observations. It was decided that a p-value of P<0.05 would represent a significant change unless specifically noted in the text. Values given in the text refer to means with standard errors (S.E.).
RESULTS
1. Effects of GABA on the Response of Blood Pressure in the Rats
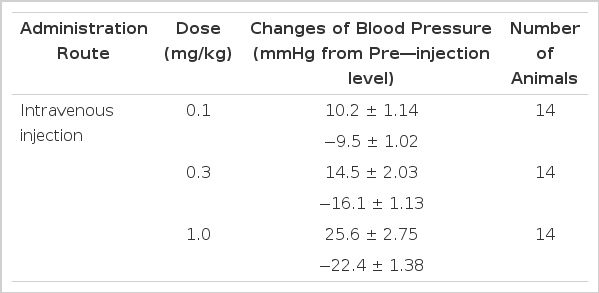
The rats were allowed to stabilize for at least 30 min. before experimental protocols were initiated. When cardiovascular parameters became stabilized in a steady-state condition, GABA at given doses was injected into a femoral vein of the rat, resulting in a dose-dependent fall in blood pressure, followed by a secondary elevation as shown in Table 1.
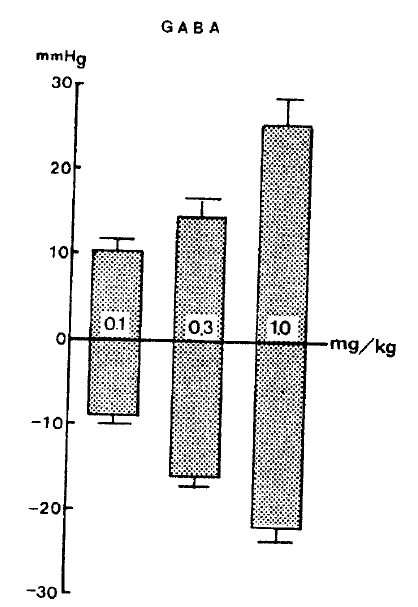
In 14 rat experiments, GABA at 0.1, 0.3 and 1.0 mg/kg administered intravenously produced a dose-dependent reduction in arterial pressure by −9.5±1.02, −16.1±1.13 and −22.4±1.38 mmHg from the preinjection level, respectively, followed by a secondary elevation by 10.2±1.14, 14.5±2.03 and 25.6±2.75 mmHg, respectively, figure 1 shows the changes of blood pressure evoked by GABA in rats.
These results are in disagreement with the findings of Sweet et al.,9) in which even higher intraventricular doses of GABA did not induce any changes in the blood pressure of the cats.
2. Effects of Atropine and Chlorisondamine on GABA-Evoked Changes of Blood Pressure
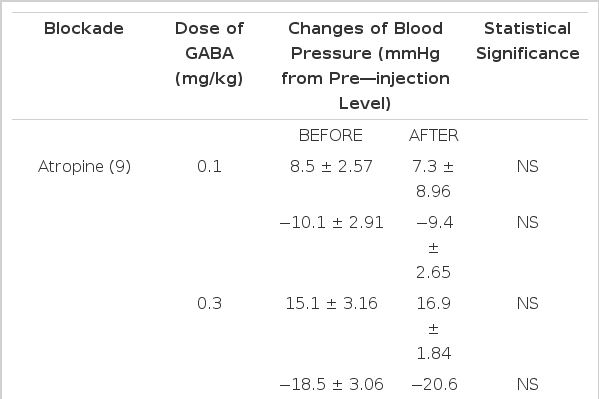
The results of this experiment are shown in Table 2. Rats were injected intravenously with 3.0 mg/kg atropine22) in order to block peripheral muscarinic receptors before administration of GABA. Preliminary studies showed that this dose of atropine blocked the vasodepressor effect evoked by muscarine. In the presence of atropine, GABA given intravenously in all of previously mentioned doses (0.1, 0.3 and 1.0 mg/kg) still produced depressor responses and also secondary pressor responses (Fig. 2).

Effects of atropine on GABA-induced changes of blood pressure of the rats. Atropine (3.0 mg/kg) was injected intravenously after obtaining the control values. Ordinate: changes of blood pressure in mmHg. Abscissa: doses of GABA in mg/kg. Numerals in the upper parenthesis indicate the number of animals used in the present work. Vertical bars denote S.E. of mean. Statistical differences were obtained by comparing the what with the corresponding control values.
As shown in Table 2, ganglionic blockade with intravenous injection of chlorisondamine22) (1.0 mg/kg) into a femoral vein of the rat did not affect the pressor responses evoked by GABA, while producing greatly decreased responses of depressor activity evoked by GABA.
In nine rats, the changes of blood pressure at 0.1, 0.3 and 1.0 mg/kg, i.v. of GABA before administration of chlorisondamine were −10.1±0.97, −19.4±2.77 and −20.3±2.31 mmHg of depressor responses, with 9.4±1.18, 13.5±1.78 and 18.1±2.04 mmHg of pressor responses, respectively. But the depressor responses evoked by GABA after chlorisondamine were greatly attenuated by −5.3±1.19 (P<0.05), −9.6±2.03 (P<0.05) and 7.4±1.72 (P<0.01) mmHg, respectively, without any changes of pressor responses. Figure 3 shows the effects of chlorisondamine on GABA-evoked changes of blood pressure.
3. Effects of Prazosin and Debrisoquin on GABA-Evoked Changes of Blood Pressure
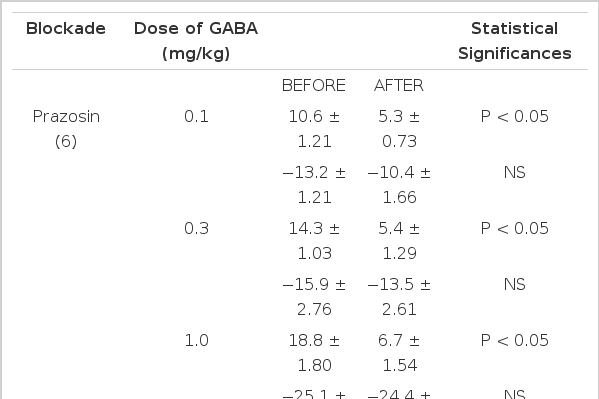
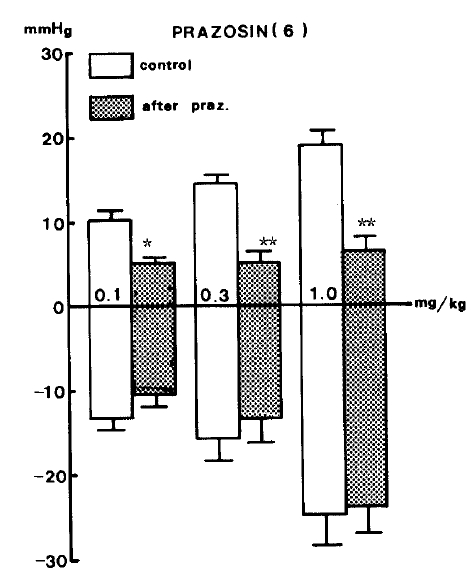
Prazosin22–24) (1.0 mg/kg), a drug presently employed in treating hypertension, was given intravenously in order to block adrenergic alpha-receptors and produced a marked reduction in pressor responses evoked by GABA but did not influence the depressor responses of it as shown in Table 3.
There were significant decreases in pressor responses of GABA by 5.3±0.73 (P<0.05), 5.4±1.29 (P<0.01) and 6.7±1.54 (P<0.01) mmHg, respectively, from the control values of 10.6±1.21, 14.3±1.03 and 18.8±1.80 mmHg, respectively, at 0.1, 0.3 and 1.0 mg/kg, i.v. of the given doses of GABA. Figure 4 represents the effects of prazosin on GABA-evoked changes of blood pressure.
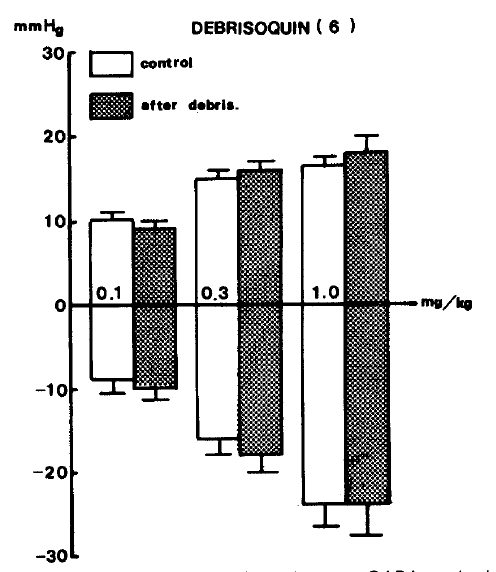
On the other hand, debrisoquin22) (3.0 mg/kg), an adrenergic neuron blocker used as an anti-hypertensive drug, was injected intravenous but did not reveal any changes to the responses of blood pressure evoked by GABA as shown in Table 2. Figure 5 indicates the effects of debrisoquin on GABA-evoked changes of blood pressure.
4. Effects of Picrotoxin and Diazepam on GABA-Evoked Changes of Blood Pressure
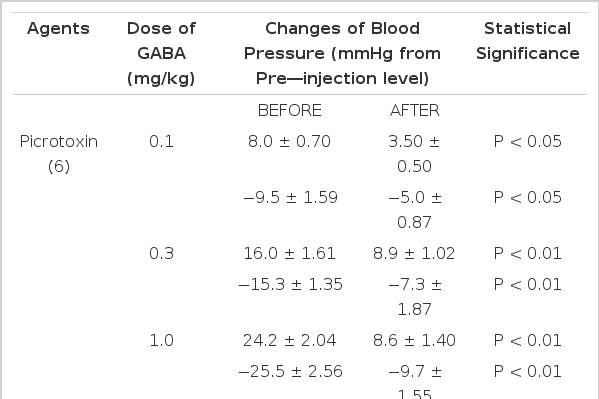
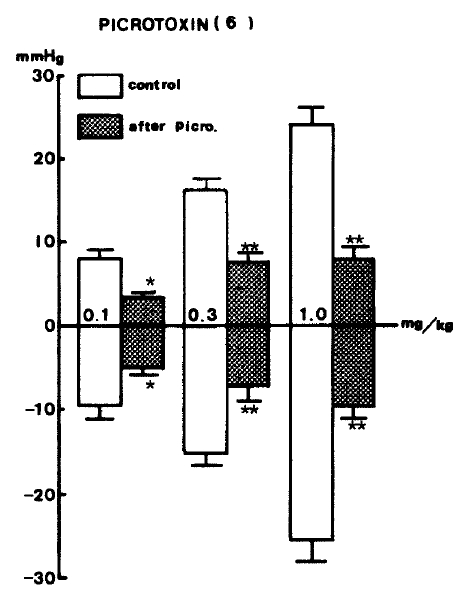
In order to examine the influence of picrotoxin on GABA-evoked changes of blood pressure, picrotoxin22,25–27) (1.0 mg/kg), a GABAergic receptor antagonist, was administered intravenously and caused a significant reduction in GABA-evoked depressor and pressor responses (Table 4).
GABA-evoked depressor activities at 0.1, 0.3 and 1.0 mg/kg, i.v. after picrotoxin were reduced by −5.0±0.87 (P<0.05), −7.3±1.87 (P<0.01) and −9.7±1.55 (P<0.01) mmHg from the control values of −9.5±1.59, −15.3±1.35, −25.5±2.56 mmHg before picrotoxin. GABA-induced pressor responses at given doses after picrotoxin were cleary diminished by 3.50±1.50 (P<0.05), 8.9±1.02 (P<0.01) and 8.6±1.40 (P<0.01), respectively, from each control of 8.0±0.7, 16.0±1.61 and 24.2±2.04 mmHg before picrotoxin. Figure 7 shows the effects of picrotoxin on GABA-evoked depressor and pressor responses.
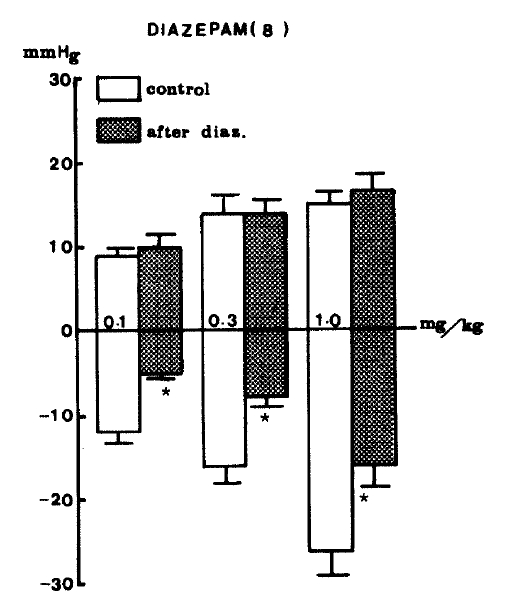
Effects of diazepam on GABA-evoked changes of blood pressure, diazepam (2.0 mg/kg) was given intravenously after obtaining the control value. The methods and other legends are the same as in Fig. 8.
In general, it is known that benzodiazepines are believed to act at specific binding sites which are closely linked to GABA receptors.22,29–32) The binding of benzodiazepines increase the affinity of the GABA receptor for GABA, thereby enhancing GABAergic transmission. In the present study, it was particularly interesting to test the effects of diazepam on GABA-evoked changes of blood pressure. Diazepam (2.0 mg/kg) administered intravenously produced a marked reduction in depressor activity evoked by GABA but did not affect secondary pressor activity evoked by GABA as shown in Table 4. Changes of blood pressure evoked by GABA at doses of 0.1, 0.3 and 1.0 mg/kg i.v. caused depressor responses by −11.9±1.84, −16.2±2.69 and −26.1±2.80 mmHg, respectively. However, after treatment with diazepam, those evoked by GABA at given doses were significantly reduced by −5.3±0.61 (P<0.05), −8.1 ±1.65 (P<0.05) and −16.4±2.55 (P<0.05) mmHg, respectively, from each control value. Secondary elevation of blood pressure evoked by GABA was still present even in the presence of diazepam as shown in Figure 7.
DISCUSSION
The present experimental results suggest that GABA produces a biphasic response: depressor followed by a secondary pressor response, and that this hypotensive activity may be exerted through the stimulation of GABAergic receptors and hypertensive activity due to the activation of adrenergic alpha-receptors, which appear to be associated with the GABAergic receptor.
In terms of the fact that GABA-evoked depressor responses were clearly inhibited by pretreatment with chlorisondamine,22) an autonomic ganglionic blocker, it is thought that the possible site of GABA-evoked depressor activity may be the autonomic ganglia or more higher center. Krantis and Kerr33) have suggested that GABA acts at an enteric neuron serving to release acetylcholine in a chloride-dependent manner. GABA also has been shown to depress transmission associated with a depolarization in mammalian autonomic ganglia.34,35) Williford et al.12) showed that vagotomy had no effect on the response evoked by muscimol, whereas stellate ganglionectomy prevented the decrease in heart rate without altering the effect of muscimol on blood pressure. In the present experiment, the portion of the autonomic nervous system responsible for the hypotensive effect of intravenons GABA is the sympathetic nervous system.
This was shown in this study by noting the absence of any modifying effect of GABA-evoked hypotensive activity by atropine and abolition of the response by pretreatment with chlorisondamine. It seems that GABA-evoked hypotensive action may be associated with the inhibition of autonomic ganglia. Furthermore, in the present work the findings that GABA-evoked depressor activity was markedly attenuated in the presence of picrotoxin, a GABAergic antagonist, indicate that the site of GABA’s effect may be GABAergic receptors mainly present in the brain.
Some investigators have shown that GABA administration directly into brain ventricles9–11,36,37) or applied onto the ventral surface of the medulla36) or onto localized sites in the medial reticular formation10) reduced blood pressure and heart rates. By recording the electrical activity in a renal nerve, it was confirmed that the cardiovascular responses were the result of reduced central sympathetic outflow. Furthermore, the effects of GABA or the GABA agonist, muscimol, were reversed by the GABA antagonist, bicuculline.11)
Guertzenstein37) has also suggested that both glycine and gamma-aminobutyric acid act to suppress vasomotor tone emanating from the caudal medulla. Similarly, in the present study, Elliott and Hobbiger15) claimed that i.v. injection of GABA into anesthetized cats reduced blood pressure in large part by a peripheral mechanism. Drugs known to interfere with the function of GABA such as picrotoxin, which inhibits the effect of GABA on chloride conductance,27) and bicuculline, which blocks the GABA receptor38), increase central sympathetic outflow and arterial pressure.39,43) These agents also increase parasympathetic outflow and decrease heart rate.40–42) Similarly, a drug that inhibits the synthesis of GABA in the CNS [thiosemicarbazide]44) and an agent that has been found to block the release of GABA [tetanus toxin]45) increase sympathetic outflow.46,47)
Conversely, substances that presumably act by directly stimulating GABA receptors (e.g., GABA and muscimol) decrease sympathetic outflow and arterial pressure and heart rate.11,18,29)
The definition of these GABA-receptor types depends on their differing agonist and antagonist specificities48): GABAA-receptor sites are bicuculline-sensitive and coupled to a chloride (Cl−) ionophore, while GABAB-receptor sites are bicuculline-insensitive and Cl−-independent48–49). Furthermore, although GABA itself is both a GABAA-and GABAB-receptor site agonist, these receptors are distinguishable in that, among others, 3-amino-1-propanesulphonic acid (3APS) is a GABAA-receptor site agonist only, whereas baclofen (b-(p-chlorophenyl)-GABA) is solely a GABAB-receptor site agonist lacking any GABAA-receptor mediated actions.48,50,51) Baclofen is stereospecific for GABAB-receptor sites, causing a reduction in transmitter output in both the peripheral and central nervous systems.48,52,54)
In view of the fact that GABA stimulates neurones which serve to both contract and relax the smooth intestinal muscle,33) by acting through bicuculline-sensitive GABAA-receptor sites coupled to a Cl− inophore, and antagonized by picrotoxinin,25–27) it is felt that GABA may interact with GABAA-receptor to induce depressor activity. Moreover, diazepam considerabley blocked the GABA-evoked hypotensive effect in the present work. It is also apparent that this finding is enough to support GABA-receptor is a site of depressor action evoked by GABA.
In support of this idea, it is known that barbiturates and benzodiazepines exert no effect on either the response to GABAB site activation or the binding of ligands to these sites.28) By contrast, hypnotic anti-anxiety drugs enhance the binding of ligands to GABAA sites and the response to receptor activation.29–32)
On the other hand, the fact that GABA-evoked depressor activity was not blocked by pretreatment with atropine, prazosin or debrisoquin suggests that hypotensive activity of GABA is not associated with cholinergic stimulation, alpha-adrenoceptor blockade, or adrenergic neuron blockade.
In light of the fact that pressor responses evoked by GABA were markedly diminished by pretreatment with prazosin or picrotoxin, it is thought that intravenous GABA causes secondary hypertensive effects through adrenergic α1-receptor stimulation, which is apparently associated with the activation of the GABAergic receptor.
The fact that the vasodepressor effect evoked by GABA was clearly reduced by pretreatment with prazosin,22) a postsynaptic alpha 1-adrenergic blocker in the blood vessels, demonstrates that GABA acts by interfering with the peripheral sympathetic function. The superiority of prazosin over other alpha-receptor blocking drugs is attributed to its greater affinity for postsynaptic receptors than for presynaptic alpha2-adrenergic receptors. Since prazosin exerts little action on presynaptic receptors, norepinephrine concentrations do not increase, and reflex sympathetic activity (for example, tachycardia) is less likely to occur.22–24)
Among the drugs which interfere with the peripheral sympathetic function, alpha adrenoceptor blocking agents alone cause reversal of the epinephrine pressor response.55)
When epinephrine is administered to untreated animals, its alpha agonist properties predominate resulting in a rise in mean arterial pressure. However, in the presence of alpha-adrenoceptor blockade, the peripheral B2-agonist properties of epinephrine predominate, and a fall in arterial pressure or a reversal of the pressor response is observed.
In contrast, the pressor responses to norepinephrine are impaired by alpha-adrenoceptor blockade but are not reversed,56) since this agent possses little B2-agonist activity.57)
Thus, the inhibitory effect of prazosin in response to secondary pressor action evoked by GABA may be taken to present specific adrenergic alpha1-receptor stimulation. Interestingly, diazepam did not inhibit GABA-evoked secondary pressor effects in our experiment. The mechanism of this hypertensive effect seems to be different from that of the hypotensive effect evoked by GABA, although it is clearly attenuated in the presence of picrotoxin.
However, it is felt that a GABA-evoked pressor effect is not associated with muscarinic action, release of catecholamine from adrenergic nerve endings or activation of autonomic ganglia, because this effect of GABA was unaffected by pretreatment with atropine, debrisoquin or chlorisondamine.