Autoradiographic and Immunohistochemical Study on the Proliferative Kinetics of Intestinal Metaplasia
Article information
Abstract
In order to elucidate the proliferative behavior of the intestinal metaplasia around gastric cancer, the authors used both in vitro tritiated thymidine (3H-thymidine) autoradiography and in vivo bromodeoxyuridine (BrdUrd) immunohistochemistry for labeling the proliferative cells of the normal pyloric glands and metaplastic gastric glands.
The results of the methods were comparable: The labeling pattern and the rate of labeling were very similar. In the normal pyloric mucosa, the labeled cells were confined to the isthmus region, indicating that pyloric glandular cells are normally renewed from the isthmus region. On the other hand, a zone of the labeled cells was found in the lower half of the intestinalized mucosa, indicating that cell proliferation took place deep in the mucosa, just like the case of normal intestinal glands. The labeling indices of the pyloric mucosa were 19.4% by autoradiography and 18.0% by immunohistochemistry, and that of the intestinalized gastric glands were 25.2% by autoradiography and 24.2% by immunohistochemistry.
In conclusion, both 3H-thymidine autoradiography and BrdUrd immunohistochemistry showed that the proliferative kinetics of the intestinalized gastric glands was similar to that of the normal intestinal glands rather than the pyloric glands, i.e. a lower level of proliferative zone and higher labeling index were present.
INTRODUCTION
The intestinal metaplasia of the stomach, which may be associated with a change in the direction of differentiation from the normal pyloric pattern to that of the intestine, is often accompanied by gastric cancer1,2) and so considered to be a precancerous condition3,4,5). However, it also has been regarded as a normal repair mechanism6) because it accompanied by peptic ulcer or chronic gastritis1,2).
In order to understand the meaning of the metaplastic change of the gastric mucosa around the gastric cancer, it is necessary to elucidate the proliferative characteristics of the intestinal metaplasia. As to the site of cell proliferation in the intestinalized gastric mucosa, there are two different sets of data. Winawer and Lipkin7) report 3H-thymidine incorporating cells concentrated at the middle level and even located at the surface of the mucosa. On the other hand, Hattori and Fujita8) demonstrate a zone of labeled cells in the lower one-thrid of the mucosa.
Bromodeoxyuridine (BrdUrd), like 3H-thymidine, is incorporated into the DNA of the proliferating cells. So an immunohistochemical method using monoclonal antibodies to BrdUrd can be used to study the proliferative kinetics of the gastric mucosa. So far, however, BrdUrd immunohistochemistry was mainly used to study the proliferative kinetics of human tumor9,10).
In this study, the authors used both 3H-thymidine autoradiography and BrdUrd immunohistochemistry to elucidate the proliferative behavior of the inestinal metaplasia found in the pyloric mucosa around the gastric cnacer.
MATERIALS AND METHODS
1. Autoradiographic Study
The materials used in this study were biopsy specimens taken from 9 patients suffering from advanced gastric cancer (Table 1). The specimens were carefully taken from the nondiseased portions of the pyloric mucosa, and 3 biopsy specimens were obtained from each individual. 3H-thymidine autoradiography was performed using the in vitro method described by Hattori and Fujita8). Briefly, the specimens were cut into 1 mm pieces and washed in sterile saline solution. They were then immersed in MEM (modified Eagle’s medium) supplemented with 10% fetal calf serum containing 20 μCi of 3H-thymidine (methyl-3H-thymidine, Amersham, England, specific activity, 25 μCi/mmol) per 1 ml and incubated for 60 min at 37°C. After fixation with 10% formalin, they were dehydrated in a graded alcohol series and embedded in paraffin. The embedded tissues were carefully oriented and sectioned serially and longitudinally to the long axis of the glandular tubule. Sectons of about 2–3 μm in thickness were mounted on glass slides treated with tissue adhesives, dipped in SAKURA NR-M2 nuclear emulsion, and developed in FD-111 after 6 weeks’ exposure. They were stained with hematoxylin and eosin.
The in vitro labeling of the tissues with 3H-thymidine did not provide constant results. Frequently, only the margins of the specimens were labeled with 3H-thymidine. Sometimes, however, we were successful in labeling a whole layer of the specimens in which a zone of the labeled epithelial cells was found. In this study, we used these specimens in order to interpret cell proliferation kinetics in the pyloric and intestinalized mucosa.
2. Immunohistochemical Study
The materials used in the BrdUrd immunohistochemical study were resected stomachs from 4 patients suffering from advanced gastric cancer (Table 1). Informed consent for administration of BrdUrd was obtained from each patient. The method used in this study is described by Hoshino et al9). Briefly, the patients were given a 60-min i.v. infusion of BrdUrd, 200 mg/m2, at the time of surgery but before gastric resection. Non-cancerous and non-ulcerated regions of the antral mucosa were cut into rectangular pieces and embedded in paraffin. They were cut serially and mounted on glass slides treated with tissue adhesives. Tissue sections were deparaffinized for 15 min in xylene, rinsed 3 times (5 min each time) in 0.01 M phosphated buffered solution (PBS), hydrated for 30 min in 1N HCl, and incubated for 20 min at 37°C in PBS containing 0.05% proteinase type VII (Sigma, U.S.A). The sections were then stained by avidin biotin peroxidase method using Vectastain ABC kit: they were incubated for 16 hr at room temperature with 1 : 60 dilution of purified anti-BrdUrd monoclonal antibodies (Bection Dickinson, U.S.A) in bovine serum albumin, for 30 min at room temperature with biotinilated antimouse IgG (horse); and for 30 min at room temperature with ABC. The tissue sections were rinsed with PBS at each stage. Finally, the slides were reacted with 0.03% 3,3′-diaminobenzidine (DAB) and PBS containing 1% H2O2. Light counter stains were performed by hematoxylin.
3. Analysis of Results
Black-silver grains in the nuclei (Fig. 1) and dark brown colored nuclei (Fig. 2) were considered as labeled cells in autoradiography and immunohistochemistry, respectively, and a zone of labeld cells was considered as a proliferative zone of the mucosa8,11). The site of the labeled cells was indicated by the number of counted nuclei from the surface of the gland. The labeling index (LI) was defined as the percentage of the labeled cells counted in a total number of over 1,000 nuclei in the generative cell zone between the level of the uppermost labeled cells and that of the lowermost cells in each specimen8).
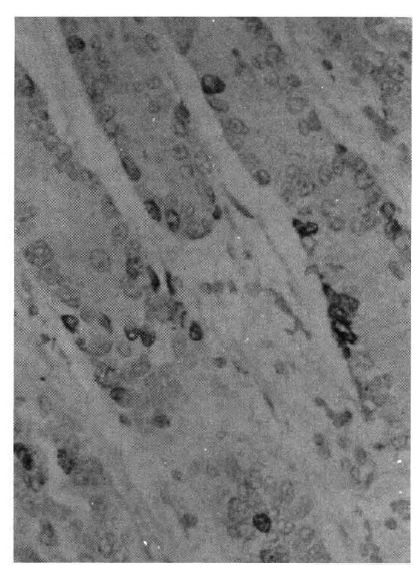
Representative presentation of labeled cells by in vitro 3H-thymidine autoradiography. Black-silver grains indicate the incorporation of 3H-thymidine into the DNA. H.E. X 200.
RESULTS
The schematic representation of the results of this study is shown in Fig. 3. In short, the metaplastic gastric glands showed a lower level of proliferative zone and a higher LI. The difference was statistically significant (p<0.01, Table 2).
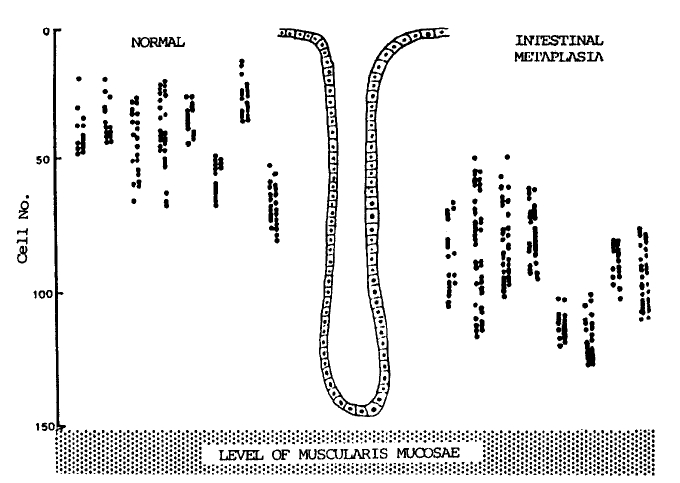
A schematic representation of the sites and distribution of labeled cells in a specimen from a 46-year-old male, obtained by in vivo bromodeoxyuridine immunohistochemical method. The site of labeled cells was indicated by the number of nuclei counted from the surface of the gland. The labeling index, the percentage of the labeled cells counted in a total number of over 1000 nuclei in the generative cell zone between the level of the uppermost labeled cells and that of the lowermost cells in each specimen in this case was 19.4% in the normal pyloric glands and 22.0% in the intestinalized glands.
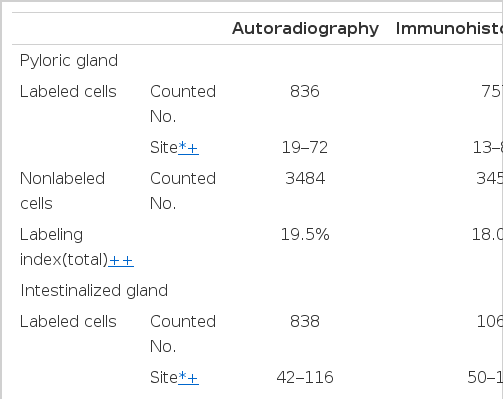
Comparison of Labeling Sites and Labeling Indices (Lls) of Pyloric and Intestinalized Glands Studied by in Vitro 3H-thymidine Autoradiography and in Vivo Bromodeoxyuridine (BrdUrd) Immunohistochemistry
1. Autoradiography
Autoradiographs of the pyloric mucosa not involved with metaplastic change showed the labeled cells to be confined to the region of the isthums between the base of the pits and the upper part of the pyloric gland (the middle one-third of the mucosa) (Fig. 4). The cell order, the number of counted nuclei from the surface of the gland of the labeled cells, was distributed between 19 and 72 (Table 2). The LI varied from 15.3 to 26.6%, and the total LI was 19.4% (Table 3).
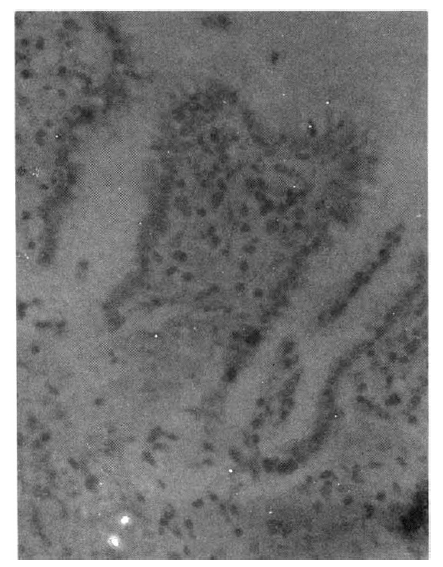
Autoradiograph of normal pyloric mucosa (from a 62-year-old male) obtained by in vitro incubation with 3H-thymidine for 60 min. Labeled epithelial cells are confined to the region of the isthmus of the pyloric gland. H.E. X 200.
In the autoradiographs of the intestinalized mucosa, a zone of the labeled epithelial cells was found in the lower third of the mucosa (Fig. 5). The cell order of the labeled cells of metaplastic glands was distributed between 42 and 116 (Table 2). The LI varied from 20.6 to 29.4%, and the total LI was 25.2% (Table 3).
2. Immunohistochemistry
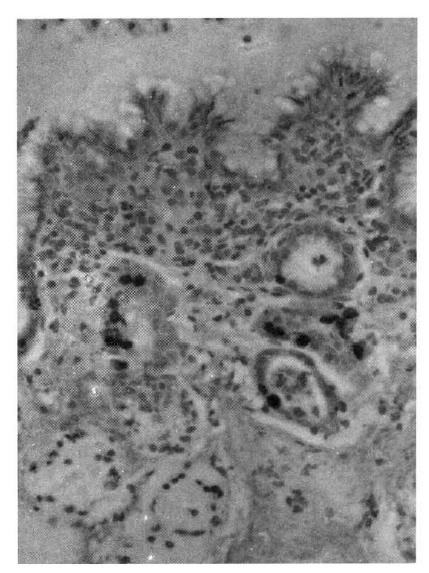
The pattern of distribution of labeled cells revealed by in vivo BrdUrd immunohistochemistry (Fig. 6a, 6b, and 7a, 7b) was very similar to that revealed by in vitro 3H-thymidine autoradiography.

Photomicrograph of normal pyloric mucosa (from a 46-year-old male) obtained by hematolxylin-eosin stain (6A) and in vivo bromodeoxyuridine immunohistochemical method (6B). Labeled epithelial cells are confined to the isthmus region of the gland (6B). X 100.

Photomicrograph of intestinalized mucosa (from a 46-year-old male) obtained by hematoxylin-eosin stain (7A) and in vivo bromodeoxyuridine immunohstochemcial method (7B). Labeled cells are seen at the lower level of the mucosa (7B). X 100.
The cell order of the labeled cells in the pyloric mucosa varied from 13 to 84 and in the intestinalized mucosa between 50 and 135 (Table 2). The LI varied from 12.5 to 22.2% in the pyloric mucosa and between 21.8 and 27.2% in the intestinalized mucosa. The total LI of the pyloric mucosa was 18.0% and that of the intestinalized mucosa 24.2% (Table 3).
DISCUSSION
Tritiated thymidine autoradiography is widely used to study cellular kinetics of the gastrointestinal mucosa. Although 3H-thymidine has been administered intravenously in a limited number of patients7,11,12), a systemic administration of the isotope is almost impossible in humans because of the potential radiation hazard to normal tissue13). So the only acceptable method of obtaining specimens is through the “tissue or organ culture” method. In the present study, the specimens were labeled by brief in vitro incubation with 3H-thymidine. But application of this technique still has problems: It is tedious to perform and requires several months to complete.
Recently, Gratzner14) developed a monoclonal antibody that can identify nuclei containing BrdUrd. Anti-BrdUrd monoclonal antibody can be detected by immunohistochemical methods15). This is an important breakthrough for studies of cell kinetics. BrdUrd, like 3H-thymidine, is incorporatd into nuclear DNA during DNA synthesis but is neither radioactive nor myelotoxic at the doses to be used for in vivo labeling studies15,16). The immunohistochemical method of detecting anti-BrdUrd immunohistochemistry was mainly used to evaluate the proliferative activity of human tumors and then to elucidate the prognosis of individual patients9,10), but application of this method to cell kinetic study of intestinal metaplasia is rare.
In the present study, the authors used both in vitro 3H-thymidine autoradiography and in vivo BrdUrd immunohistochemistry for elucidation of the proliferative kinetics of the intestinal metaplasia found around the gastric cancer. The results of both methods were comparable: The labeling pattern and the rate of labeling were very similar.
Both methods revealed that the labeled cells were confined to the middle third (isthmus region) of the mucosa in the normal pyloric mucosa and to the lower third of the mucosa in the intestinalized gastric mucosa. This labeling pattern indicates that in the human pyloric mucosa the cells proliferate within the isthmus region and that cell proliferation takes place deep in the mucosa in the intestinalized mucosa. It is generally accepted that in mammalian gastrointestinal mucosa, proliferation of epithelial cells is confined to a certain level of the mucosa: In the stomach, it is a neck area11,17) and in the intestine it is a lower crypt18,19). Therefore the proliferative pattern of intestinalized gastric glands is just like that of normal intestinal glands.
However, the cell order of the labeled cells counted from the surface of the gland studied by BrdUrd immunohistochemistry was higher (i.e., labeled cells were located deeper in the gland) than that obtained by 3H-thymidine autoradiography. This difference might be due to the difference of the method itself or to the materials used. Hattori and Fujita8) point out that the generative cell zone of the metaplastic gland shifts from the isthmus to the base of the gland following atrophy of the pyloric gland. So the difference of the cell order might be due to the difference of the materials (i.e., degree of intestinalization) rather than to the difference of the method itself, because the difference between the LIs calculated by the 2 methods were statistically insignificant.
The LI of the intestinalized mucosa was significantly higher than that of the pyloric mucosa. This can be explained by the fact that the renewal time of the intestinal epithelium is faster than that of the gastric epithelium18,19). Winawer and Lipkin7) calculated an LI using in vivo 3H-thymidine autoradiography in a patient and report that the LI of a normal gastric gland is 14% and that of a metaplastic gland 19%. Hattori and Fjuita8) report that the LI of a normal pyloric gland varies from 20 to 25% and that of am intestinalized gastric gland from 28 to 34%. Hattori20) reports that the LI of a normal pyloric gland varies from 16 to 26% and the LI of an intestinalized gland 12 to 26%. In the present study, the LI of the normal pyloric mucosa was 19.4% by in vitro 3H-thymidine autoradiography and 18.0% by in vivo BrdUrd immunohistochemistry. The LI of the intestinalized gastric mucosa was 25.2% by autoradiography and 24.2% by immunohistochemistry.
Recently several investigators noted that the intestinal metaplasia of the stomach can be divided into several subtypes, and the significance as a precancerous condition is confined to certain sybtypes21–25). In the present study, chemical subtyping of the intestinal metaplsia was not done. Morphologically, most of them were complete types showing Paneth cells. The difference of proliferative kinetics among the different subtypes of intestinal metaplasia requires further investigation.
In conclusion, BrdUrd immunohistochemistry is expected to be used widely in the fields of cell kinetics because of its advantage over 3H-thymidine autoradiography: in vivo use and its capacity of rapid processing with very similar results to autoradiography. Both methods showed that the proliferative kinetics of intestinalized gastric glands was similar to that of normal intestinal glands rather than pyloric glands, i.e., a lower level of proliferative zone and higher LI were present.