A Case of Intestinal Hemorrhage Due to Small Intestinal Metastases from Primary Lung Cancer
Article information
Abstract
Although intestinal metastases from lung cancer are not rare at postmortem studies, the development of clinically significant symptoms from the gastrointestinal metastases is very unusual. We report a case of small intestinal hemorrhage leading to intestinal perforation secondary to metastases from a large cell carcinoma of the lung in a 31-year-old man along with a review of the literature.
INTRODUCTION
It has been well known that about half of the patients with lung cancer have metastases at the time of initial diagnosis. The common sites of distant metastases are the bone, liver, adrenal glands, and lymph nodes1). The clinical symptoms of the metastases of bone, liver, and lymph nodes usually occur relatively earlier. Therefore, these metastases are often diagnosed at the same time the diagnosis of lung cancer was made. However, in the case of metastases to the kidneys, adrenal glands, gastrointestinal tract, and skin, the clinical symptoms of metastasis do not appear early and are usually found at postmortem examination2). In particular, the intestinal metastases of primary lung cancer are hardly diagnosed until the development of intestinal obstruction, perforation or hemorrhage; these cases usually show high mortality rate on operation.
Since Morgan’s first report in 1961 of small intestinal metastasis from lung cancer, 27 cases with the clinical symptoms of perforation, obstruction and/or hemorrhage have been reported. Among them, only 1 case was intestinal hemorrhage1,3–13). We here present a case of intestinal hemorrhage that progressed to intestinal perforation and caused by small intestinal metastasis of carcinoma of the lung, large cell type.
CASE REPORT
A 31-year-old Korean male patient was transferred to our hospital from a local hospital because of persistent melena of undetermined origin. Four months ago, the patient visited the hospital with complaints of chest tightness and productive cough. An X-ray film of the chest at that time showed a patch infiltration in the right lower lung field. Studies of the sputum for acid fast bacilli were negative but anti-tuberculosis medications were started under the impression of pulmonary tuberculosis. Three months later, he was admitted to the same hospital because of melena and underwent extensive work-ups including gastroscopic examination, barium studies of the colon and small bowels, and a gastric mucosa scan for Meckel’s diverticulum, but no bleeding focus was found. During the stay in that hospital, the melena improved spontaneously and he was discharged. He did fairly well until 10 days prior to this admission when recurrent melena developed. Additional studies including 99mTC-sulfur colloid scintigraphy were carried out, but no bleeding focus was found.
On admission, the patient appeared pale and acutely ill. The blood pressure was 130/70 mm/Hg, pulse rate 120/min, respiratory rate 20/min, and the temperature 36.5°C. The heart sound was normal without murmur. Breathing sound was decreased at the right lower lung field. No lymphadenopathy was found in the neck or axilla. No palpable abdominal mass was felt. Rectal examination revealed internal hemorrhoid and hematochezia.
The hemoglobin was 4.6 g/dl and the hematocrit was 14%. The white blood cell count was 6300/mm3 and the platelet 203,000/mm3. The urea nitrogen was 22.4 mg/dl, the creatinine 1.5 mg/dl, the protein 2.5 g/dl (the albumin 1.3 g/dl and the globulin 1.2 g/dl), the bilirubin 0.4 mg/dl, the alkaline phosphatase 53 IU, the aspartate aminotransferase (AST) 10 IU, and the alanine aminotransferase (ALT) 10 IU. The serum sodium was 127 mEq/L and the potassium 4.1 mEq/L. Chest X-ray film revealed an ill defined dense infiltrate with air bronchogram in the right lower lung field (Fig. 1). Mesenteric arteriography was performed but could not find any bleeding focus. Because of severe hematochezia, the flexible sigmoidoscopy was not available. The hemorrhoid with massive bleeding was found by anoscopy. An emergency hemorrhoidectomy was carried out on the second hospital day. However, a large amount of hematochezia persisted even after the operation and signs of serious disseminated intravascular coagulopathy (DIC) follwed. On the 10th hospital day while the DIC improved, the patient complained of abdominal distension and pain. Ascites was suspected.

Posteroanterior chest X-ray shows ill defined dense infiltrates with air bronchogram in the right lower lobe.
An abdominal paracentesis revealed intraabdominal bleeding. An emergency exploratory laparotomy was perfomed. On operation, multiple polypoid masses of various sizes were palpated intraluminally in the jejunum from 10 cm below the Treitz ligament to the 120 cm distally. A large perforation of the jejunum (8 cm in diameter) probably caused by necrosis of the bowel wall was found on the antemesenteric border of the jejunum 20–25 cm below the Treitz ligament. Multiple mass lesions including hematomes were also found in the mesenteric root of the small intestine. Small bowel resection and end-to-end anastomosis were performed. On section, 36 polypoid masses were scattered grossly along the full length of the resected jejunum. The largest mass was 5.0×8.0×4.0 cm in size and composed of brownish homogeneous materials. Similar materials were filled in the mesenteric arteries and veins (Fig. 2).
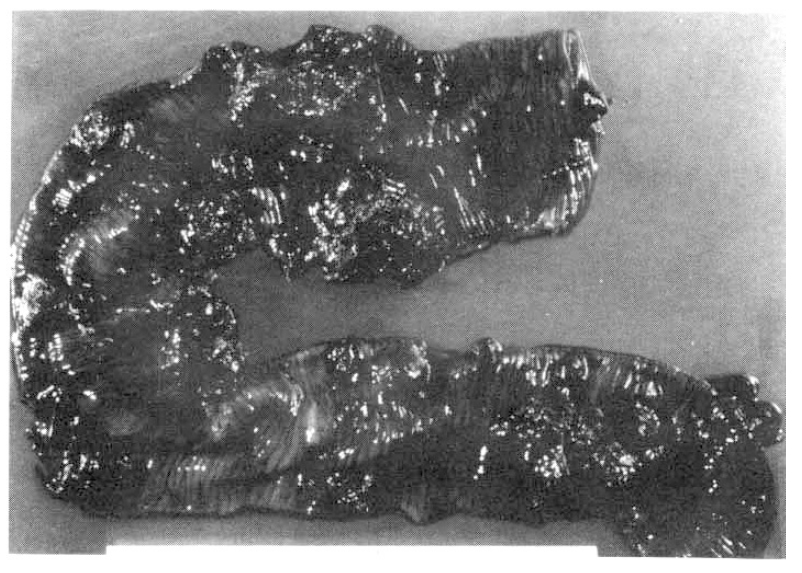
Gross finding of mucosal surface. Thity-six dark brown polypoid masses are scattered along the full length of the small intestine. The largest one is 8.0×5.0×4.0 cm in size.
Mlicroscopically, numerous hemosiderin pigments were scattered throughout the fields. The cells in the polypoid mass lesion were consistent with large round cells of undetermined origin. Each cell had a round nucleus containing prominent nucleoli. The cytoplasm was homogenous and clear (Fig. 3). The immunohistochemical study for S-100 protein was negative. Electron microscopically, obvious desmosomes were identified as convincing evidence of carcinoma (Fig. 4). Bronchoscopic examination, which was done on the 10th postoperative day, revealed an obstructing round mass at the entrance of the right main bronchus (Fig. 5). Histologically, this lesion was diagnosed as large cell carcinoma of the lung (Fig. 6).

The polypoid tumor of the intestine is composed of large round cells without pigment. The cells are relatively uniform in size.(H&E, ×100)

Bronchoscopic biopsy specimen. The tumor cells are large round cells with cellular pleomorphism.(H&E, ×200)
On the 27th postoperative day, massive amounts of hematemesis and hematochezia developed again. The patient died on the 31st postoperative day due to hypovolemic shock.
DISCUSSION
It has been reported that 41% of all patients with lung cancer are found to have evidence of distant metastases at the time of initial diagnosis, but the clinical symptoms of the metastases to the gastrointestinal tract are rarely present3). The distant metastases of lung cancer are usually found at the lymph nodes, brain, bone, adrenal glands, and liver through lymphatic spread2,14). The gastrointestinal metastases occurred through the vertebral venous plexus1,7).
The frequency of small intestinal metastases from lung cancer depends on its histopathologic type. McNeill et al. reported that primary lung cancer metastases to the small intestine was mainly from large-cell type carcinoma as our case1).
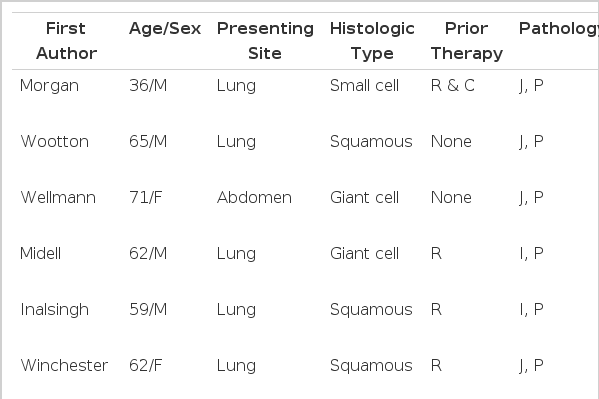
The peak incidence was in the sixth and seventh decade, similar to those of patients with primary lung cancer1,3–13). The majority of cases were men1,3–13). Most of the metastatic small intestinal tumors originated from the cervix, skin, ovaries, and kidneys14). The clinical symptoms were usually those of bowel obstruction, perforation or rarely hemorrhage. By reviewing the literature of the 27 cases reported, 18 had intestinal perforation, 8 had obstruction and only 1 had hemorrhage(Table 1). Postmortem examinations of 431 lung cancer patients studied over an 11-year period(1) showed that 46 patients (11%) had metastases to the small intestine.
Among the 46 patients who received exploratory laparotomy with the symptoms and signs of intestinal obstruction or perforation, 6 patients were found to have primary lung cancer. All of these 6 patients died within 16 weeks after the operation1). On the other hand, according to the report of Arianha et al., 10% of patients with suspected metastasis were found to have benign lesions as the cause of small bowel obstruction15). Also, in the other 2 reports16,17), 25% and 38% of patients with a history of cancer who underwent exploratory laparotomy because of intestinal obstruction were found to have either a benign disease or a new primary cancer as the cause of the obstruction. According to the report by Woods IV et al, 8 out of 13 patients who underwent emergency exploratory laparotomy experienced successful palliation by surgical intervention3). These data suggested that the cancer patients with intestinal obstrcution who do not have a proven abdominal cancer should undergo exploratory laparotomy. Morgan et al. report a case of perforation caused by metastatic cancer after chemotherapy and they suggested that there might be a close relationship between the chemotherapy and perforation18). Inalsingh et al. also explained that local factors, such as trauma and/or irradiation, which could influence the immune response were related to the metastases12).
Among 17 cases with intestinal perforation, 7 presented with perfortion after chemotherapy and radiotherapy. In conclusion, with increasing use of chemotherapy and radiotherapy for the treatment of cancer, the possibility of perforation of the small intestine from the metastatic tumor should be considered more carefully than before.