Small Airway Disease in Rheumatoid Arthritis*
Article information
Abstract
Variety of pulmonary lesions are thought to be associated with rheumatoid arthritis (RA). These lesions traditionally have included pleurisy with or without effusion, Caplan’s syndrome, pulmonary rheumatoid nodules, diffuse interstitial fibrosis, and pulmonary arteritis and hypertension. But little attention has been paid to the airways in RA. Recently, several repots have suggested an association between airflow limitation and RA, but its incidence is not known. Also whether there exists a parameter of disease activity of RA, suggesting the presence of small airway disease (SAD) is not clear.
To answer these questions, the serologic parameters which reflect the disease activity of RA and pulmonary function tests which reflect small airway dysfunction were performed on 36 lifetime nonsmokers with RA who had normal chest x-ray findings. The prevalence of SAD and the relationships between the disease activity parameters of RA and pulmonary function were observed.
The results were as follows. The percentages of patients with abnormal values for diffusing capacity, frequency dependence of compliance (C1.0/C0.0), forced expiratory flow25–75%, Vmax50% and Vmax75% were 45.5%, 62.5%, 40%, 22.8% and 11.4%, respectively. There was statistically significant negative correlation between C1.0/C0.0 and ESR. But consistent correlation between other pulmonary function tests and clinical and serologic parameters of RA, and differences in pulmonary function between patients who were serologically positive and negative for CRP and FANA, were not found.
In conclusion, SAD, without the influence of smoking, is frequently associated with RA, but, the presence of SAD cannot be predicted from any clinical and seologic parameters of RA currently in use.
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic disease associated with a number of different lung disorders. These include pleurisy with or without effusion, Caplan’s syndrome, rheumatoid lung nodules, diffuse interstitial fibrosis and pulmonary hypertension due to pulmonary arteritis1–7). In the past, obstructive airway disease in RA patients was thought to be related to cigarette smoking, thus receiving little attention. But when RA patients without underlying lung disease were histopathologically proven to have obstructive bronchiolitis, people began to look at obstructive small airway disease (SAD) as a manifestation of rheumatoid lung disease8). But at this time, not much data is present on the prevalence of SAD in RA and the possibility that it may be due to other factors has not been completely ruled out. So it cannot be safely concluded that obstructive small airway dysfunction observed in RA is simply a manifestation of RA itself9,10).
We studied 36 lifetime nonsmokers with RA who had normal chest x-rays to find out the prevalence of SAD in these patients and to detect, if any, parameters that tell which of asymptomatic RA patients will need further pulmonary function tests reflecting small airway function, by examining the relationship between the pulmonary function tests and the disease activity of RA.
SUBJECTS AND METHODS
1. Subjects
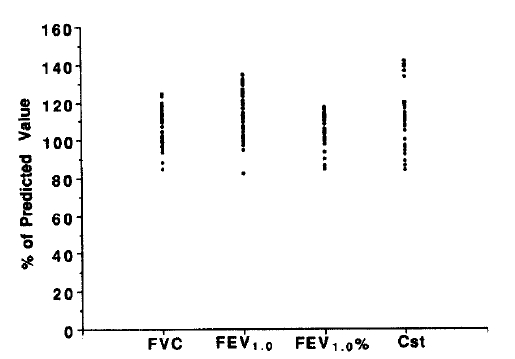
Thirty-six patients with classic or definite RA, as defined by American Rheumatoid Association criteria, who were being followed at Seoul National Univerisity Hospital’s outpatient clinic, were selected for this study. All patients had normal chest x-rays and patients with prior history of smoking, chronic pulmonary diseases like bronchial asthma, chronic bronchitis, emphysema, etc. or acute pulmonary disease within 3 months of study were excluded. All subjects also had normal predicted values for forced vital capacity (FVC), forced expiratory volume 1 second (FEV1.0), FEV1.0/FVC (%) and static compliance (Cst).
2. Methods
Detailed history taking and physical examination were done to evaluate the duration of disease, the extent of joint involvement and the duration of morning stiffness, and serologic tests such as rheumatiod factor, CRP, ESR and fluorescent antinuclear antibody (FANA) were performed.
Pulmonary function tests were performed using the CHESTAC-25 Pulmonary Function Computer System (Chest, Japan). From the flow-volume curve we obtained FVC, FEV1.0, forced expiratory flow25–75% (FEF25–75%), Vmax50% and Vmax75%. The test was repeated three times and the best results were taken. Likewise diffusing capacity (DLCO/VA) was measured three times, with the highest value chosen as his or her capacity. The compliance was measured with CSO828 FC Body Plethysmography (Autobox). The measurement for Cst was done as follows. To measure the esophageal pressure, we used an esophageal balloon catheter, a 100 cm-long polyethylene tube with internal diameter of 1.4 mm with a latex balloon 50 μm in thickness at its distal end. After anesthetizing the nasal cavity with 4% lidocaine jelly, the esophageal balloon was inserted at least 60 cm through the nasal cavity. At this length, the balloon is situated in the stomach and during inspiration it should maintain positive pressure. We then slowly pulled out the catheter and, at a certain point, the pressure reversed itself to negative during inspiration. At this point, we assumed that the balloon was at the gastroesophageal junction. After that, the catheter was pulled out for about 10 cm more to situate it where it showed biggest negative pressure during inspiration and was least influenced by the heart beat. The patient was asked to perform a Valsalva maneuver to eliminate all the air present in the catheter. Then, through the syringe connected to a 3-way stopcock, 8 cc of air was injected into the catheter and then 7.8 cc was removed so that only 0.2 cc of air remained. He or she was then instructed to take a maximum inspiration to total lung capacity and breathe out slowly. By using curves taken during this procedure, static compliance was obtained between functional residual capacity and functional residual capacty±500 ml. The dynamic compliance was measured while the patient was breathing in increasing frequencies from 12 to 72 breaths per minute in 5 stages. Great care was taken to maintain constant tidal volume. Inspiratory values for 5 breaths were calculated and the average taken as dynamic compliance for that respiration rate. From these values frequency dependence of compliance, ratio of C1.0 (dynamic compliance at respiration rate of 60 breaths per minute) and C0.0 (dynamic compliance at respiration rate of O breaths per minute) was calculated. All of the values above, except for C1.0/C0.0, were expressed as percentages of predicted values for those age, sex, height, weight, barometric pressure and temperature so as to minimize the error of comparing the measured value directly. The normal predicted values were derived from Baldwin et al.11). for FVC, Berglund et al.12) for FEV1, Schmidt et al.13) for FEF25–75%, Cheniack et al.14) for Vmax50% and Vmax75% and Hellisen et al.15) for Cst. Parameters FEV1, FVC, FEV1/FVE, FEF25–75%, and Cst were said to be abnormal if they were less than 80% of predicted value, Vmax50%, Vmax75%, and DLCO/VA were said to be abnormal if less than 75% of predicted value and C1.0/C0.0 were said to be abnormal if C1.0 was less than 80% of C0.016,17).
RESULTS
1. Characteristics of Patients
Table 1 shows the summary of the clinical data. Four males and thirty-two females were included in the study and their ages ranged from 23 to 67 years old with the average of 45. The duration of disease differed from 1 year to 17 years. Each patient had involvement in 3 to 14 joints at the time of the study as evidenced by either pain or swelling. The duration of morning stiffness varied from 1 to 8 hours. Twenty-eight of thirty-six patients studied were RA factor positive with titers of 1:40 to 1:2,580. ESR ranged from 3 to 99, CRP negative to 6 (+). FANA was positive in 18 patients with a titer of more than 1:40.
All of the patients had normal FVC, FEV1, FEV1/FVC (%) and Cst values (Fig. 1).
2. Diffusing Capacity and the Parameters of Small Airway Disease
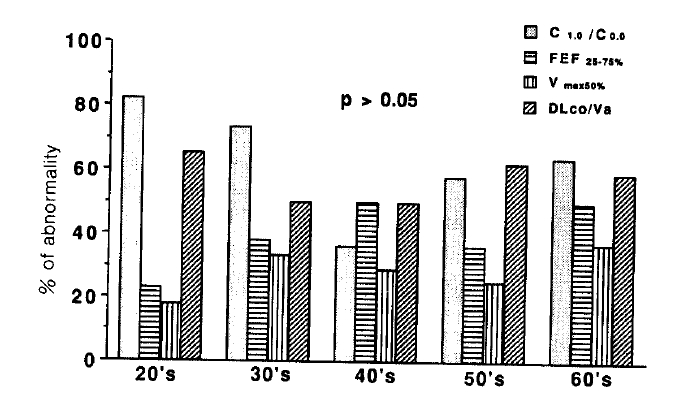
The percentages of patients with abnormal values for C1.0/C0.0, FEF25–75%, Vmax50% Vmax75% and DLCO/VA were 62.5%, 40%, 22.8%, 11.4% and 45.5% respectively (Fig. 2). There were no significant correlations between age and parameters of pulmonary function tests (Fig. 3).

Profile of parameters of small airway dysfunction and diffusing capacity.
*values represent simple %, not % of predicted value
3. Relations between Pulmonary Function Tests and Clinical and Serologic Parameters
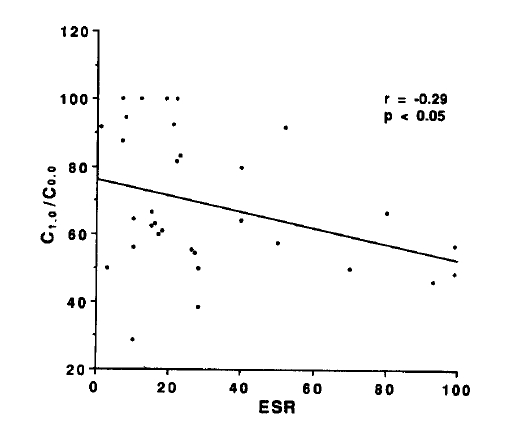
The values for DLCO/VA, FEF25–75%, Vmax50%, Vmax75% and C1.0/C0.0 did not correlate with duration of disease, titer of RA factor and duration of morning stiffness, and differences in pulmonary function tests between positive and negative reactors for CRP and FANA were not observed (data not shown). The ESR and C1.0/C0.0 had statistically significant negative correlation with C1.0/C0.0 decreasing as ESR increased (Fig. 4).
DISCUSSION
In the past, pulmonary function tests were not performed routinely in RA patients and, if performed, they were usually done on patients with interstitial lung disease18–20). Some studies excluded patients with evidence of airway obstruction from their studies, and the possibility of airway obstruction was either overlooked21) or was attributed to smoking. So the exact prevalence of airway obstruction in RA patients is not known. Colins et. al.22) found airway obstruction in 26 out of 43 RA patients studied, but 24 of those 26 patients had a history of smoking, thus not completely ruling out smoking as the cause for the obstruction in that group. But in 1977, Gedds et. al.8) reported six patients with rapidly progressive airway obstruction in the absence of chronic bronchitis or emphysema. Five of the six patients had classical rheumatoid arthritis and pathologically bronchioles with diameter of 1–5 mm were obstructed with mural scar tissue suggesting that airway obstruction may be a manifestation of RA itself. Later, the prevalence of airway obstruction in 100 RA patients with normal chest x-rays was found to be 32% and the values for FEV1, FVC, FEV1/FVC and FEF25–75% in these patients were significantly lower than those of the normal control suggesting that airway obstruction may be the most common pulmonary disease in RA patients9). To completely rule out the influence of cigarette smoking on airway obstruction, clinical manifestations, serial pulmonary function tests and pathologic findings of six RA patients who were nonsmokers and had had evidence of airway obstruction for the past 10 years, were reviewed23). It showed that the RA patients developed airway disease regardless of smoking, that the airway disease was very diverse and that the deterioration of pulmonary function was much more rapid than smokers with chronic obstructive pulmonary disease. Other connective tissue disease patients without respiratory symptoms also show abnrmalities in their pulmonary functions with each disease having its own patlern of lung dysfunction. Progressive systemic sclerosis predominantly shows evidence of large airway obstrution, systemic lupus erythematosis decreased diffusing capacity and rheumatoid arthritis small airway disease24).
On the other hand, in many comparative studies with normal controls, increased incidence of airway obstruction was not found in RA patients, and in cases where obstruction was observed they were attributed to specific etiologies like smoking, denying the possibility that the obstruction was due to RA itself10,25,26).
Why are there so many conflicting reports on obstructive SAD in RA? There are several reasons. First, these contradictory results may have been obtained because different studies were performed on different populations. SAD may be caused by smoking, air pollution, drugs, infection and α1-antitrypsin deficiency. These factors may have influenced the outcome of each of these studies. In our study, to minimize the effects of these factors, we restricted the study population to persons that reside in the same geographic area, without history of chronic lung disease, not having or had within 3 months of study acute pulmonary illnesses and having normal chest x-rays. Penicillamine and gold which are used in the treatement of RA are known to cause small airway obstruction27). We were not able to gather accurate drug history in all of the patients in this study, thus we are unable to fully exclude the possibility that drugs used in the past might have influenced the results of this study. Secondly, the parameters used to detect SAD were not the same in all of the studies. There are many parameters which are thought to reflect small airway dysfunction; FEF25–75%, closing volume, volume of isoflow, Cdyn, Vmax50%. Vmax75%, and so on. But there is no sine qua non of small airway disease and each test has its own limitations and sensitivity16,17,28–31). We used C1.0/C0.0 which was confirmed by others and also by us32) to be a sensitive parameter of small airway involvement, but we also measured FEF25–75%, Vmax50%, and Vmax75% to overcome the problems arising from using only one parameter. The third reason may be the difference in normal ranges of pulmonary function tests used in the studies. If we have too wide a normal range, its usefulness in detecting asymptomatic patient with abnormal pulmonary function is impaired33). Thus, the best way to determine the normal range is by each laboratory repeatedly testing healthy normal subjects and setting their own guidelines. In our study, on the basis of our laboratory’s results32), we used over 80% of predicted value for FEF25–75% and over 75% of predicted value for Vmax50% and Vmax75% as the normal ranges.
The results of our study suggest that SAD may well be present in RA patients, irrespective of smoking, since large percentages of nonsmoking RA patients had abnormal values for tests indicative of SAD (C1.0/C0.0 62.5%, FEF25–75% 40%, Vmax50% 22.8% and Vmax75% 11.4%).
Although the exact mechanism for SAD in RA patients is not clear, several hypotheses have been proposed. As mentioned above, MS phenotype of α1-antitrypsin30), penicillamine27), viral infection9) and autoimmune exocrinopathy34) have been proposed as possible etiologic factors. There were linear depositions of IgG in the alveolar walls of RA patients with acute bronchiolitis, showing evidence that immunologic factors might be involved in the pathogenesis of this disease35). SAD in RA patients may be considered as a secondary phenomenon to interstitial pulmonary fibrosis, but in this study there were no significant correlations of DLCO/VA and parameters of small airway disease (data not shown), so it will be hard to explain the decrease in small airway disease parameters as a secondary phenomenon to interstitial fibrosis. Although not confirmed, the pathogenesis of SAD may be multi-factorial. Smoking, drugs, viral infection, autoimmune factors, and many other factors not yet determined, may all in some complex way interact to obstruct the small airways in a genetically-predisposed RA patient.
If we can predict the presence of SAD from conventional tests of disease activity used clinically, it will be helpful in deciding which of the asymptomatic RA patients will need pulmonary function tests. Previous studies have shown that RA patients with small airway disease have significantly higher positive rates for FANA34), and that serologically positive patients have significantly lower diffusing capacity than sero-negative patients36). This suggested that there may be some relationships between the dysfunction in pulmonary function and the duration and severity of illness and rheumatoid factor titers, but these correlations were not statistically significant37). In our study, only ESR and C1.0/C0.0 showed statistically significant negative correlation with all other parameters having no association (data not shown). So, it will be difficult to know whether or not a patient has SAD with conventional parameters of RA disease activity now in use.
Definite confirmation of SAD in RA patients must be backed up by histologic findings. To perform an open lung biopsy in an asymptomatic patient in unrealistic but transbronchial lung biopsy may be an alternative. Also, there are reports that cell profile of BAL (bronchoalveolar lavage) fluid may give insight to the leions of peripheral bronchus38) in RA patients. So, to understand the true incidence and pathogenetic mechanisms of SAD in RA patients, we think histologic backup by bronchoscopy is necessary.
Summarizing, we performed tests on pulmonary function and serologic markers of disease activity in 36 nonsmoking RA patients to observe the prevalence of SAD and the relationship between the disease activity parameters of RA and pulmonary function tests. The percentages of patients with abnormal values for C1.0/C0.0, forced expiratory flow25–75%, Vmax50%, Vmax50%, Vmax75% and diffusing capacity were 62.5%, 40%, 22.8%, 11.4% and 45.5% respectively. Statistically significant negative correlation was observed for C1.0/C0.0 and ESR, but other parameters did not show significant correlations with each other. So, SAD is found in RA rather frequently but the presence of SAD cannot be accurately predicted from any of the clinical or serologic parameters of RA currently in use.
CONCLUSION
We conclude that SAD is frequently associated with RA, but the presence of SAD cannot be predicted from any clinical or serologic parameters of RA and to understand the true incidence and pathogenetic mechanisms of SAD in RA patients, we think histologic backup by methods including bonchoscopic examination is necessary.
Notes
This Study was supported in part by a grant from Japan Adult Disease and Clinical Memorial Foundation.