Mycobacterium fortuitum Peritonitis Associated with Continuous Ambulatory Peritoneal Dialysis
Article information
Abstract
Runyon group IV atypical mycobacteria, Mycobacterium fortuitum, is an environmental organism and is capable of producing a variety of clinical infections such as cutaneous infection, abscess and pulmonary and ocular infection. Rarely, it has been a documented cause of peritonitis in patients receiving continuous ambulatory peritoneal dialysis (CAPD). We report a case of M. fortuitum peritonitis in a patient undergoing CAPD and emphasize the importance of mycobacterial cultures in patients with CAPD-associated peritonitis whose routine culturing yields no organisms repeatedly.
INTRODUCTION
Peritonitis is the most common and serious problem in patients receiving continuous ambulatory peritoneal dialysis (CAPD) and is the major cause for transfer to other methods of dialysis1). The vast majority of cases result from infections with aerobic gram-positive (60% to 70%) or gram-negative organisms (20% to 30%)2,3). Anaerobic bacteria, fungi and mycobacteria account for less than 10% of isolates cultured4). Although mycobacterial infections are uncommon, they occur ten times more frequently in patients with end-stage renal disease (ESRD) than in patients with normal renal function because of impaired cellular immunity5). Mycobacterial infections are also more likely to be extrapulmonary and result from non-tuberculous organisms in ESRD patients5).
Mycobacterium fortuitum is rapid growing, non-tuberculous mycobacteria and it has been isolated as a pathogen from several extrapulmonary sites. Few cases of M. fortuitum causing peritonitis in patients receiving CAPD have been reported6–8).
We report a case of CAPD-associated peritonitis caused by Mycobacterium fortuitum.
CASE REPORT
A 54-year-old woman was admitted to the hospital in May 1992 with a chief complaint of diffuse abdominal pain for a duration of one week. She had been treated with CAPD since January 1992 for end-stage renal disease of unknown etiology. One week before admission, the patient developed diffuse abdominal pain, fever and chill and she was treated with intraperitoneal cefazolin and tobramycin for six days at the out-patient department. At that time, the peritoneal fluid contained 2,250 leukocytes per mm3 with 85% polymorphonuclear cells, and routine culture yielded no organisms. In spite of six days’ antibiotics therapy, there was no improvement.
The temperature was 37.8°C, the pulse rate was 87 per minute and the respirations were 25 per minute. The blood pressure was 140/90 mmHg. On physical examination, the patient was alert and appeared acutely ill. Examination of the head and neck was normal, except for pale conjunctiva. The heart and lungs were normal. The abdomen was rigid and diffusely tender but the catheter exit site showed no signs of infection or leakage; the liver and spleen were not palpable. There was peripheral edema. The peripheral leukocyte count was 9,000/mm3, with 88% neutrophils, 8% lymphocytes, 4% monocytes and hemoglobin was 7.5 g%. Her serum sodium was 137 mEq/L, potassium 4.2 mEq/L, chloride 106 mEq/L, calcium 8.3 mEq/L, phosphate 2.7 mEq/L, urea nitrogen 27 mg/dl and creatinine 6.2 mg/dl. Serum total protein was 6.0 g/dl, albumin 2.9 g/dl, total bilirubin 0.3 mg/dl, aspartate aminotransferase 17 IU/L, alanine aminotransferase 3 IU/L and alkaline phosphatase 39 IU/L. X-ray films of the chest and abdomen were normal.
The peritoneal fluid was cloudy and contained 1,000 leukocytes per mm3, with 90% polymorphonuclear cells. Intraperitoneal ceftizoxime and amikacin were administered after routine culturing of the peritoneal fluid was done. Initial gram and acid-fast staining revealed no organisms and routine cultures showed no growth. Abdominal pain was persistent and routine cultures again showed no growth, but follow-up acid-fast staining was reported to be positive. Therefore, cultures for mycobacteria were requested repeatedly. Antibiotics were discontinued and antituberculous drugs (isoniazid, rifampin, ethambutol and streptomycin) were begun. The peritoneal catheter was surgically removed and hemodialysis was begun.
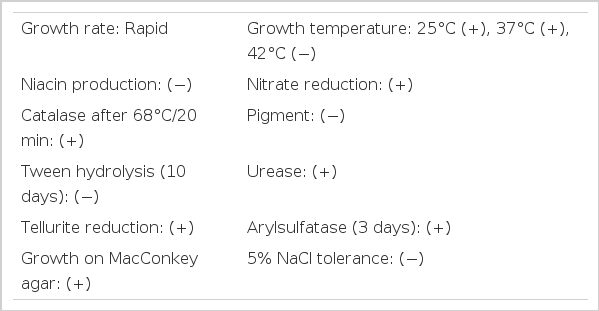
Atypical mycobacteria, not Mycobacterium fortuitum, grew three times after 2 weeks of incubation periods. Colonies of atypical mycobacteria were sent to Korean Institute of Tuberculosis, Korean National Tuberculosis Association for identification and susceptibility tests, and they were identified as M. fortuitum. The results of the identification test are given in Table 1. The result of susceptibility test against antituberculous drugs showed “all resistance”. Test against antimicrobial agents was not performed. Empiric therapy with intravenous use of ceftizoxime and amikacin was started and the patient improved. Until now, she has done well on hemodialysis.
DISCUSSION
Mycobacterium fortuitum is the commonest of the group IV (Runyon’s classification) rapidly growing, non-tuberculous mycobacteria and is readily isolated from a number of natural sources such as soil, dust and water9). This organism displays low-inherent pathogenicity but, when inoculated into a sterile body site, such as through accidental trauma or surgery or through the CAPD catheter into the peritoneum, may cause serious infection. This has been identified as a cause of sporadic post-surgical infections, as well as of outbreaks of nosocomial disease that involve dialysis6–8), specific types of surgery10,11) and catheter-related bacteremias12). Patients with end-stage renal disease have an increased susceptibility to mycobacterial infection5), so mycobacteria account for “routine culture-negative” peritonitis in CAPD patients partially.
The route of CAPD peritonitis due to M. fortuitum is likely through contamination of the peritoneal dialysis catheter lumen or the exit site, since there are no reported cases of spontaneous peritonitis unrelated to peritoneal dialysis2). This is opposed to tuberculous CAPD peritonitis, which usually follows reactivation of a latent tuberculous focus13).
There are a number of difficulties in the diagnosis of infections with M. fortuitum. The problem in the recovery of these organisms is that they grow slower than common bacterial pathogens, usually requiring a minimum of three to five days to produce visible growth on primary isolation. Thus, they go undetected when culture media are discarded after two days, as is common practice in routine cultures of CAPD fluid. In additon, colonies of M.fortuitum resemble diphtheroids on blood agar and may be mistaken for them on gram stain, unless acid-fast stains are also obtained14,15). Longer observation of routine peritoneal fluid cultures, early use of mycobacterial media and increased familiarization with the gram stain appearance of non-tuberculous mycobacteria are necessary for proper diagnosis2). In our case, acid-fast staining of peritoneal fluid was requested several times and, in the result, “positive for acid-fast bacilli” gave an important clue for identifying the causative organism.
Treatment of infections due to M. fortuitum has some difficulty. M. fortuitum is resistant in vitro to all of the first-line and most of the second-line antituberculous drugs, and M. fortuitum isolated from our patient also showed in vitro resistance. Because some commonly used antibiotics such as amikacin, cefoxitin, doxycycline and sulfonamides show sensitivity to M. fortuitum16), proper selection of antibiotics is important, but antimicrobial susceptibility test against mycobacteria is not always routinely performed. Successful treatment depends on early removal of the peritoneal catheter12), drainage of fluid collections and appropriate selection of antibiotics based on antimicrobial sensitivity tests. And, in order to reduce the risk of acquired drug resistance, use of two drugs such as amikacin and cefoxitin in combination is recommended in the initial therapy17). Although we did not have information about antimicrobial susceptibility, we started empirical therapy with intravenous use of ceftizoxime and amikacin and removal the peritoneal catheter. Thus, the patient improved.
In practice, mycobacterial cultures should be obtained in all patients with CAPD-associated peritonitis who have negative routine cultures, and also fail to respond to empirical antibiotic therapy3). The peritoneal fluid total WBC count, as well as the differential WBC count, is not helpful in the diagnosis of the majority of the cases of mycobacterial peritonitis, complicating peritoneal dialysis2,13).
In summary, we reported a case of peritonitis caused by M. fortuitum in a CAPD patient whose routine peritoneal fluid cultures showed no organisms repeatedly, and who failed to respond to proper empirical antibiotic therapy. This serves to emphasize the importance of mycobacterial cultures in patients with CAPD-associated peritonitis whose routine culturing yields no organisms repeatedly.