Cimetidine Improves the Accuracy of Creatinine Clearance as an Indicator for Glomerular Filtration Rate
Article information
Abstract
Background:
Although endogenous creatinine clearance is often used as an indicator for the glomerular filtration rate (GFR), it may result in an overestimation due to its tubular secretion. Since cimetidine is known to inhibit tubular secretion of creatinine, it may improve the accuracy of the creatinine clearance in measuring GFR.
Methods:
Creatinine clearance (Ccr) was compared with iothalamate clearance (C10th) during oral administration of either placebo or cimetidine in 25 patients with varying degrees of renal dysfunction.
Results:
Cimetidine itself had no effect on C10th but decreased Ccr, improving its validity, as measured by a significant decrease of Ccr/C10th from 1.72 during placebo to 1.17 during cimetidine administration. The degree of overestimation measured by the Ccr was more pronounced in those with more severe renal dysfunction. A significant inverse correlation was noted between Ccr/C10th and GFR. No apparent side effect due to cimetidine was noted.
Conclusions:
These results suggest that cimetidine improves the accuracy of Ccr as an indicator for GFR in patients with varying degrees of renal dysfunction.
INTRODUCTION
A great deal of evidence indicates that endogenous creatinine clearance (Ccr) is inadequate for measuring glomerular filtration rate (GFR) in patients with renal diseases1–8). Ccr measures GFR with a mean overestimation by 23–96%9–14). Furthermore, the degree of overestimation varies greatly from one patient to another and even in the same individual over time11,15).
It has been shown that tubular secretion of creatinine increases as renal function decreases, especially in patients with glomerular disorders8–10). The overestimation seen in patients with renal diseases appears to be due to hypersecretion of creatinine by injured renal tubular cells11,16). To circumvent these problems, while GFR can be measured with the aid of “true filtration markers” such as insulin, iothalamate, diethylenetriaminepenta-acetic acid and ethylenediaminetetra-acetic acid, these are less convenient in handling and more costly than creatinine.
Secretion of creatinine by the tubular transport system can be inhibited by several anionic and cationic substances17). Among them, cimetidine has been known to reduce secretion of creatinine without impairing GFR, improving the accuracy of Ccr as a marker for GFR12,18,19). However, previous studies simultaneously measured iothalamate clearance (C10th), as a standard GFR, and Ccr during cimetidine treatment. Therefore, it may have not ruled out a possible effect of cimetidine on C10th.
The present study was aimed to investigate the effect of cimetidine on the Ccr. Ccr was measured during administration of either placebo or cimetidine. C10th was simultaneously determined to serve as standard GFR.
MATERIAL AND METHODS
1. Patient Selection
The study was performed in 25 patients with varying degrees of renal dysfunction, who were selected from inpatient population at the Division of Nephrology, Chonnam University Hospital. Informed consent was obtained from each patient. Ccr pre-estimated according to the Cockroft and Gault formulation20,21) less than 10 mL/min was excluded. Other exclusion criteria were pregnancy, use of H2-receptor antagonist or antacids, allergy to cimetidine, use of drugs which can influence the metabolism of cimetidine or can interfere with creatinine secretion, and unstable renal function.
2. Study Protocols
The patients were instructed to follow a 3-day schedule to take either placebo or cimetidine. They were supine-positioned throughout and had food and fluid intake every 4 h. The amount of fluid taken was 20 mL/kg, to ensure sufficient flow of urine and to prevent tubular reabsorption of creatinine. Coffee and smoking were not permitted. They were allowed to sleep from 11 : 00 pm to 08 : 00 am.
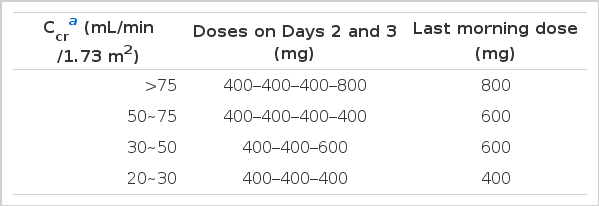
The dose of cimetidine was adjusted according to the degree of renal dysfunction in an attempt to achieve approximately equal blood cimetidine levels (Table 1). To obtain maximal inhibition on the tubular creatinine secretion, the maximally permitted oral daily dose of 2,000 mg was used in case of normal renal function.
On Day 1, they were given placebos four or five times. A 24-hour urine was collected through the Foley catheter from 08 : 00 am on Day 1 to 08 : 00 am on Day 2. No clearance measurement was done. On Day 3, 24-hour urine from 08 : 00 am to 08 : 00 am on the next day was collected.
C10th as standard GFR was measured between 10 : 00 am and 2 : 00 pm on Days 1 and 3 using the 24 h urine.
For the measurement of C10th, 125I-iothalamate was continuously infused (20 μCi/h, for 3 h) following a priming dose of 50 μCi as bolus. Blood samples were obtained from the arm vein opposite to that used for the iothalamate infusion at 90, 120, 150, and 180 min of infusion. Plasma creatinine or 125I-iothalamate concentrations at the beginning and at the end of each clearance period (30 min) were averaged. Satisfactory urinary output during these periods was maintained by replacing the urinary loss noted in the preceding clearance period with an oral load of water.
125I-iothalamate was counted in a gamma scintillation counter (Hewlett Packard, Cobra 5000). Creatinine concentrations were measured by a modified Jaffé reaction in an autoanalyser (Impact 400E). C10th was determined by averaging values obtained from three clearance periods. The Ccr was computed as mL/day and converted into a mL/min.
Clearances were corrected for the body surface area. Ccr/C10th was calculated to learn to what extent Ccr overestimates GFR.
Whole blood counts, liver function tests and analysis of the urinary sediment were done to monitor possible side-effects of cimetidine.
Data are expressed as means±SD. To compare the renal function parameters during administration of placebo and cimetidine, paired t-test was applied. Linear regression was used to study the relation between Ccr/C10th and GFR. A P-value smaller than 0.05 was considered statistically significant.
RESULTS
The subjects (12 men, 13 women) aged 41–23 years ranging between 18 and 65. Their types of renal disorders were chronic glomerulonephritis (4 cases), diabetic nephropathy (4 cases), nephrotic syndrome (3 cases), autosomal dominant polycystic kidney disease (2 cases), hypertensive nephrosclerosis (1 case), lupus nephritis(1 case), miscellaneous causes (2 cases) and unknown (4 cases).
Cimetidine had no effect on GFR measured as C10th, being 44±27 and 44±29 mL/min/1.73m2, during the placebo and during cimetidine treatment, respectively (Table 2). However, cimetidine resulted in a significant decrease of Ccr, from 69±36 during the placebo to 50±31 mL/min/1.73m2 during the cimetidine. The difference between Ccr and C10th decreased from 24±16 to 5±4 mL/min/1.73m2.
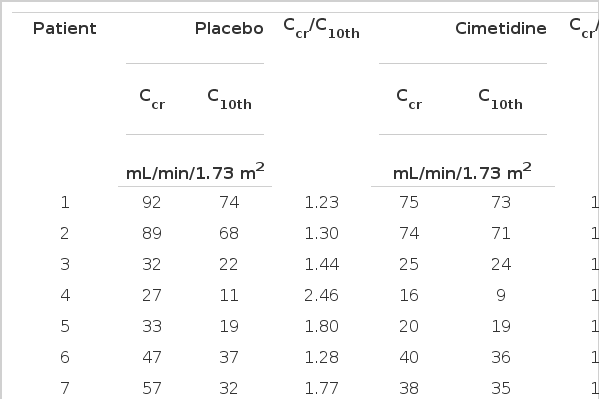
Creatinine Clearance (Ccr), Iothalamate Clearance (C10th) and Ratio of Ccr to C10th During Placebo and Cimetidine Administration
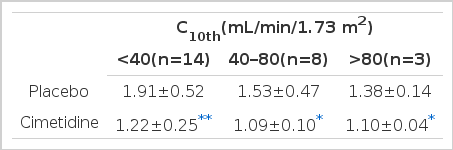
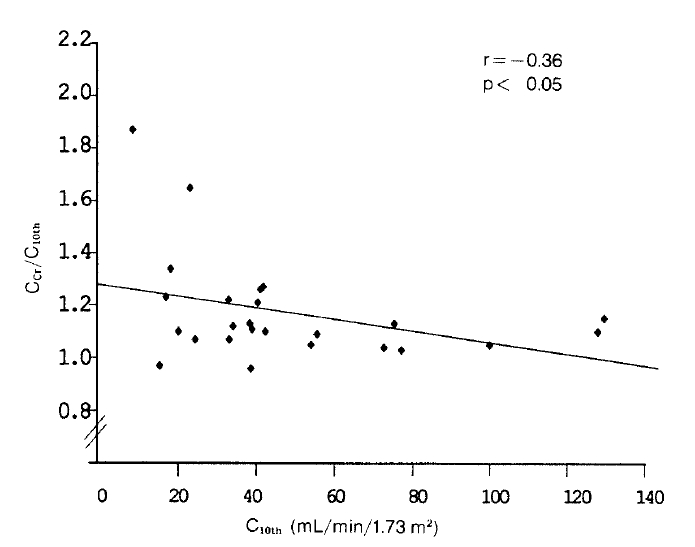
Ccr/C10th also significantly decreased from 1.72±0.51 to 1.17±0.20, indicating an improvement in validity (Fig. 1). When the patients were classified into three groups, according to their renal function as measured by C10th, Ccr/C10th was larger in those with more severe renal dysfunction, indicating a more pronounced overestimation of GFR (Table 3). A significant inverse relation was noted between Ccr/C10th and GFR (Fig. 2).
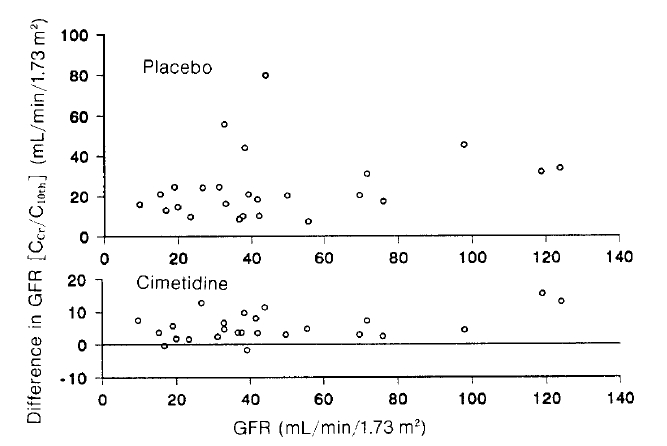
Ccr and C10th disagreed during the placebo treatment, which was improved by the cimetidine treatment (Fig. 3). No patient reported any side effect due to cimetidine.
DISCUSSION
Ccr in 25 patients with renal diseases overestimated GFR by 72%, being similar to those reported in the previous studies4,9). In addition, the degree of overestimation was larger in those with more severe renal dysfunction. A significant inverse correlation noted between Ccr/C10th and GFR, as has been shown in the previous studies1,10), suggests that the degree of overestimation is associated with the degree of renal functional impairment.
Creatinine and cimetidine are secreted by the organic cation secretary system22). The reduction of tubular creatinine secretion due to cimetidine is assumed to result from competitive inhibition on the common transport system in the proximal tubule, cimetidine having a higher affinity than creatinine for the carrier at the luminal membrane23).
Cimetidine only incompletely reduced the tubular creatinine secretion. Previous studies19,24) reported that patients with incomplete inhibition had a larger cimetidine clearance, along with lower plasma cimetidine concentration and cimetidine/creatinine ratio, than did those with complete inhibition19). This ratio is important, in that certain plasma ratio of unbound competitive compounds is necessary to induce complete inhibition25). Taken together, it may be recommended that a maximal permitted dose of cimetidine should be used to make the validity of Ccr better.
In the previous studies1,12,18,19,26,27), Ccr/C10th after administration of cimetidine varied from 0.89 to 1.35. The variation may be related to different dosage schedules and routes of administration of cimetidine. In addition, the fall of Ccr/C10th below 1.026) raises the question whether tubular reabsorption of creatinine might exist. Although tubular reabsorption of creatinine has been reported in rats28), evidence is lacking in humans.
On the other hand, no serious adverse effects due to cimetidine were noted, which may be of special importance where cimetidine is used for a non-therapeutic purpose. In a recent meta-analysis of randomized clinical trials with cimetidine, the adverse effect was negligible even with the dosage as high as 2,000 mg/day29).
The cimetidine-aided creatinine clearance can be promoted as a convenient and inexpensive as well as an exact measure of GFR. It may replace classical measurement of GFR with a true filtration marker as this is more expensive and less convenient, especially for the long-term follow-up studies of renal function30). Moreover, monitoring the progress of renal deterioration by plotting the reciprocal values of serum creatinine against time31) might become more reliable by the use of cimetidine. Finally, cimetidine may improve the sensitivity of serum creatinine in detecting minimal changes in renal function by restoring the inverse relationship between serum creatinine and GFR, which is actually blunted by the increase of tubular secretion and reduction of GFR1).
In summary, our results show that oral administration of cimetidine improves the accuracy of Ccr as a marker for GFR.
Notes
This work was supported by Clinical Research Grant (1992) of Chonnam University Hospital