Influence of Arachidonic Acid on Catecholamine Secretion in the Perfused Rat Adrenal Medulla
Article information
Abstract
The present study was conducted to investigate the influence of arachidonic acid, which is known to be an important unsaturated fatty acid component of membrane phospholipids and to be liberated by phospholipase A2 action, on secretion of catecholamines (CA) from the isolated perfused rat adrenal glands and to clarify the mechanism of its action.
Arachidonic acid (10 uM) perfused into an adrenal gland of the rat for 20 min caused a significant inhibition of CA secretion evoked by ACh (5.32×10−3 M), DMPP (10−4 M) and muscarine (10−4 M) while it did not affect that induced by excess K+ (5.6×10−2 M). Arachidonic acid, in the presence of ouabain (100 uM), an inhibitor of Na+, K+ -ATPase, also produced a marked inhibitory effect of CA secretion evoked by ACh, DMPP and muscarine but did not modify the secretory effect of excess K+. The perfusion of arachidonic acid along with indomethacin (30 uM), which is an inhibitor of cyclooxygenase, for 20 min attenuated markedly CA secretory effect evoked by ACh, DMPP and muscarine while it did not influence that by excess K+. Prostaglandin F2 alpha perfused in a retrograde direction for 20 min inhibited greatly the CA secretion evoked by DMPP but did not affect the effect evoked by excess K+. All of arachidonic acid, ouabain, indomethacin and prostaglandin F2 alpha used in the present study did not affect the spontaneous basal release of CA in the perfused rat adrenal glands.
Taken together, these experimental results suggest that arachidonic acid, as well as prostaglandin F2 alpha, cause the inhibitory action of CA secretion evoked by cholinergic receptor-mediated stimulation, but not by membrane depolarization, and also play a modulatory role in regulating CA secretion from the rat adrenal medulla.
INTRODUCTION
It has been known that arachidonic acid is released from the diglyceride by the sequential actions of diglyceride lipase and monoglyceride lipase. Phospholipase C cleaves the phosphodiester bond, resulting in the formation of a 1, 2-diglyceride1). In various secretory systems, including adrenal cortical cells2), mast cells3), pituitary cells4) and synaptosomes5), the secretion of hormones and neurotransmitters was accompanied with the release of arachidonic acid from cell membrane. In adrenal medullary cells, treated with intense electric fields6) or with digitonin7,8), the plasma membranes were made permeable to small molecules in such a way that Ca++ and adenosine triphosphate in extracellular milieu could freely enter into cytoplasm. In these cells, micromolar concentrations of Ca++ stimulated catecholamine (CA) secretion6–8) and tyrosine hydroxylase9,10). In non-permeabilized11) or digitonin-permeabilized12) adrenal medullary cells, influx of Ca++ caused the release of arachidonic acid in paralell with CA secretion. Moreover, Koda and his co-workers (1989)13) have also found that, in digitonin-permeabilized bovine adrenal medullary cells, oleic acid (cis-unsaturated fatty acids) enhances Ca++-induced CA secretion.
External addition of arachidonic acid increases the CA release in the absence, as well as in the presence, of agonist from the bovine adrenal medullary chromaffin cells14). Negishi, Ito and Hayaishi (1990)15) have shown that arachidonic acid stimulates CA release in the presence of ouabain by stimulation of phosphoinositide metabolism in a Ca++-dependent manner. Arachidonic acid, as well as tetradecanoylphorbol-13-acetate as activators of protein kinase C potentiates CA secretion evoked by Ca++16). Tachikawa and his colleagues (1990)16) clearly demonstrated that protein kinase C is not essential for the Ca++-dependent CA secretion from bovine adrenal chromaffin cells, but acts instead as a modulator. However, several reports have demonstrated that phorbol ester 12-0-tetradecanoylphorbol-13-acetate (TPA), a protein kinase C activator, stimulates the secretion of CA secretion from digitonin-permeabilized or electrically permeabilized chromaffin cells evoked by micromolar amounts of free Ca++12,21). These findings indicate that the activation of protein kinase C may participate in Ca++-dependent CA secretion from chromaffin cells. Tachikawa and his coworkers (1987)20) have reported that polymyxin B, a selective inhibitor of protein kinase C, completely inhibits CA secretion from cultured cells induced by ACh, high K+ medium, TPA or Ca++-ionophore-ionomycin.
However, there is no evidence or report available so far on the effect of arachidonic acid on CA secretion in the isolated perfused rat adrenal medulla. Therefore, in the present experiment, an attempt was made to investigate the influence of arachidonic acid on CA secretory response evoked by nicotinic, muscarinic and membrane depolarization-mediated stimulation.
MATERIALS AND METHODS
1. Experimental Animals
Mature male Sprague-Dawley rats, weighing 180–300 grams, were anesthetized with ether. The adrenal gland was isolated by the methods described previously22). The abdomen was opened by a midline incision, and the left adrenal gland and surrounding area were exposed by placing three hook retractors. The stomach, intestine and portion of the liver were not removed, but pushed over to the right side and covered by saline-soaked gauge pads and urine in bladder was removed in order to obtain enough working space for tying blood vessels and cannulations.
A cannula, used for perfusion of the adrenal gland (A), was inserted into the distal end of the renal vein after all branches of adrenal vein (if any), and vena cava and aorta were ligated. Heparine (400 IU/ml) was injected into the vena cava to prevent blood coagulation before ligating vessels and cannulations.
A small slit was made into the adrenal cortex just opposite the entrance of the adrenal vein. Perfusion of the gland was started, making sure that no leakage was present, and the perfusion fluid escaped only from the slit made in the adrenal cortex. Then the adrenal gland, along with ligated blood vessels and the cannula, was carefully removed from the animal and placed on a platform of a leucite chamber. The chamber was continuously circulated with water heated at 37±1°C (B)
2. Perfusion of Adrenal Gland
The adrenal glands were perfused by means of an ISCO pump (WIZ Co.) at a rate of 0.3ml/min. The perfusion was carried out with Krebs-bicarbonate solution of following composition (mM): Nacl, 118.4; KCl, 4.7; CaCl2, 2.5; MgCl2, 1.18; NaHCO3, 25;KH2PO4,, 1.2; glucose, 11.7.
The solution was constantly bubbled with 95% O2±5% CO2 and the final pH of the solution was maintained at 7.4±0.05. The solution contained disodium EDTA(10 μg/ml) and ascorbic acid (100 μg/ml) to prevent oxidation of catecholamine.
3. Drug Administration
The perfusions of DMPP (10−4 M) for 2 minutes and/or a single injection of ACh (5.32×10−3 M) and KCl (5.6×10−2 M) in a volume of 0.05 ml were made into the perfusion stream via a three way stopcock, and muscarine (10−4 M) was also perfused for 5 min.
In the preliminary experiments it was found that, upon administration of the above drugs, secretory responses to Ach and KCl returned to preinjection level in about 4 min, but the responses to DMPP in 8 min and and those to muscarine in 10 min. Generally, the adrenal gland perfusate was collected in chilled tubes.
4. Collection of Perfusate
As a rule, prior to each stimulation with cholinergic agonists or excess K+ perfusate samples were collected (4 min) to determine the spontaneous secretion of CA (“background sample”). Immediately, after the collection of the “backgound sample”, collection of the perfusates was continued in another tube as soon as the perfusion medium containing the stimulatory agent reached the adrenal gland. Each perfusate was collected for 4 to 8 min. The amounts secreted in the “background sample” have been subtracted from those secrected from the “stimulated sample” to obtain the net secretion value of CA, which is shown in all of the figures.
To study the effects of arachidonic acid on the sponataneous and evoked secretion, the adrenal gland was perfused with Krebs solution containing arachidonic acid for 20 min, then the perfusate was collected for a specific time period (“background sample”), and then the medium was changed to the one containing the stimulating agent and the perfusates were collected for the same period as that for the “background sample”.
5. Measurement of Catecholamines
CA content of perfusate was measured directly by the fluorometric method of Anton and Sayre (1962)23) without the intermediate purification alumina for the reasons described earlier22), using the fluorspectrophotometer (Shimadzu Co.).
A volume of 0.2 ml of the perfusate was used for the reaction. The CA content in the perfusate of stimulated glands by secretogogues, used in the present work, was high enough to obtain readings several fold greater than the reading of control samples (unstimulated). The sample blanks were also lowest or perfusates of stimulated and non-stimulated samples. The content of CA in the perfusate was expressed in terms of norepinephrine (base) equivalents. All data are presented as means with their standard errors, and the significance of differences were analyzed by Student’s paired t-test using the computer system as previously described24).
6. Drugs and Their Sources
The following drugs were used: arachidonic acid, muscarine chloride, ouabain octahydrate, indomethacin, prostaglandin F2 alpha, acetylcholine chloride, 1.1-dimethy1-4-phenyl piperazinium iodide (DMPP), norepinephrine bitartrate, (Sigma Chemical Co., U.S.A.).
Drugs were dissolved in distilled water (stock) and added to the normal Krebs solution as required, except indomethacin. Indomethacin was dissolved in dimethylsuloxide (DMSO). Concentration of all drugs used are expressed in terms of molar base.
RESULTS
1. Influence of Arachidonic Acid on Catecholamine Release Evoked by ACh, Excess K+, DMPP and Muscarine from Rat Adrenal Glands
Generally, when the oxygenated Krebs-bicarbonate solution was perfused into the rat adrenal gland for one hour, basal CA release became a steady state. Spontaneous CA release from the isolated rat adrenal gland amounted to 20.7±3.0 ng for 2 min from 12 experiments. In order to test the effect of arachidonic acid on the evoked-CA secretion, arachidonic acid (10 uM) was perfused 20 min prior to administration of various secretagogues. At that time, arachidonic acid itself did not modify basal secretion of CA (data not shown). As shown in Fig. 1, ACh (5.32×10−3 M) and excess K+(5.6×10−2 M) given in a volume of 0.05 ml into the rat adrenal gland increased CA secretion of 431.3±19.0ng and 277.5±33.6ng for 4 min, respectively. However, in the presence of arachidonic acid, ACh-evoked CA secretion was significantly attenuated to 322.5±18.6 (p<0.05, n=8) ng while excess K+-evoked CA release was not affected (270.5±30.5 ng/4 min, ns, n=6) as compared to the corresponding control secretion of CA. When perfused for 2 min through the rat adrenal glands, DMPP (10−4 M), which is known to be a selective nicotinic receptor agonist in autonomic sympathetic ganglia, produced a sharp and rapid increase in CA release. As illustrated in Fig. 2, DMPP-evoked CA secretion before the pretreatment with arachidonic acid amounted to 840±67.0 (0–4 min)ng and 202.5±33.5 (4–8 min)ng, while in the presence of arachidonic acid, which was introduced 20 min before perfusion DMPP, they were greatly reduced to 541.7±10.5 (0–4 min, p<0.001)ng and 67.5±15.3 (4–8 min, p<0.001)ng, respecitively, as compared with their control releases from 6 rat adrenal glands. When muscarine (10−4 M) was perfused into the perfusion stream, via three way stopcock for 5 min, CA secretion was increased to 306.3±4.0 (0.-5 m)ng and 181.3±7.8 (5–10 min) ng for 4 min from 6 rat adrenal glands. However, in the presence of 10 uM arachidonic acid, muscarine-evoked CA release was markedly inhibited to 181.3±27.6 (0–5 min, p<0.01) ng and 137.7±22.2 (5–10 min, p<0.05) ng, respectively, as compared to their corresponding control secretion of CA. Fig. 3 shows the inhibitory effect of arachidonic acid on muscarine-evoked CA secretion.

Effect of arachidonic acid on ACh-and excess K+-evoked catecholamine (CA) release from the isolated perfused rat adrenal glands. CA release was induced by a single injection of ACh (5.32×10−3) and excess K+(5.6×10−2 M) after perfusion with normal Krebs solution for one hour prior to initiation of the experimental protocol. “B” and “A” denote CA secretion evoked by ACh and excess KCl before (B) and after (A) proloading with 10 uM arachidonic acid for 20 min repectively. Numerals in the parenthesis indicate number of experimental rat adrenal gland. Vertical bars represent the standard error of the mean (S.E.M.). Ordinate: the amounts of CA secreted from the adrenal gland in ng. Abscissa: secretogogues. Statistical difference was obtained by comparing the control with the pre-treated group. Each perfusate was collected for 4 minutes. ACh: acetylcholine.
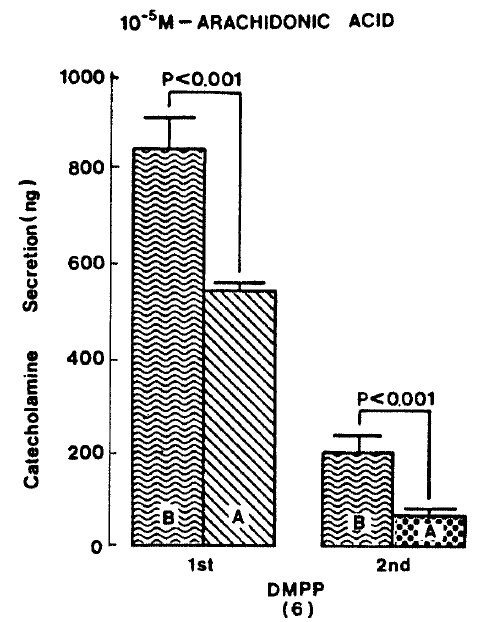
Effect of arachidonic acid on DMPP-evoked CA release. DMPP (10−4 M) was perfused into an adrenal vein for 2 min before and after pre-loading with 10 uM arachidonic acid for 20 min respectively and DMPP-induced perfusates was collected twice succesively for 4 minutes. Other legends and methods are the same as in Fig. 1.
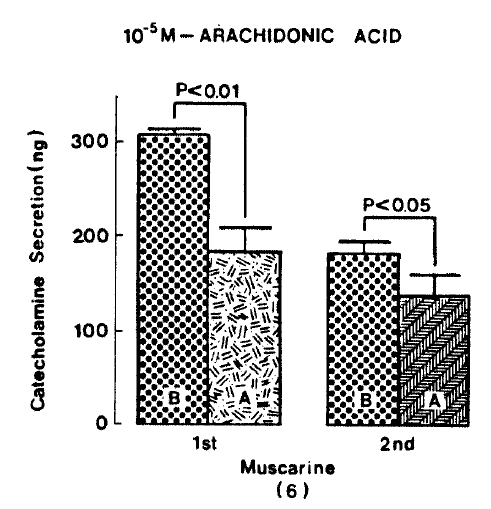
Effect of arachidonic acid on muscarine-evoked CA release from the rat adrenal glands. Muscarine (10−4 M) was perfused into an adrenal vein for 5 min and its perfusate was collected twice for each 5 minutes before and after pre-treatment with 10 uM arachidonic acid for 20 min, respectively. Other legends and methods are as in Fig. 1.
2. Influence of Arachidonic acid Plus Ouabain on CA Secretion Evoked by ACh, Excess K+, DMPP and Muscarine
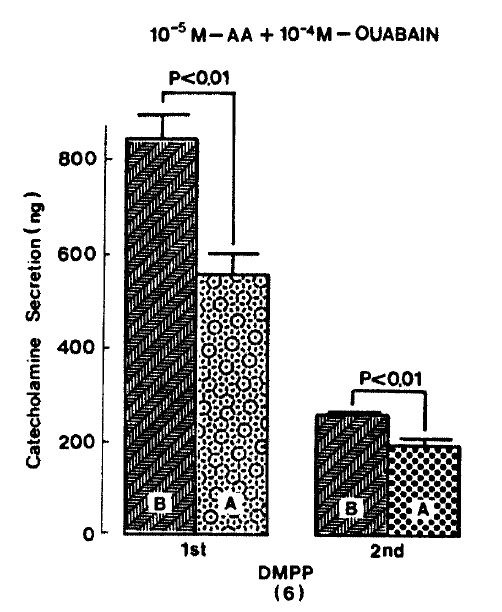
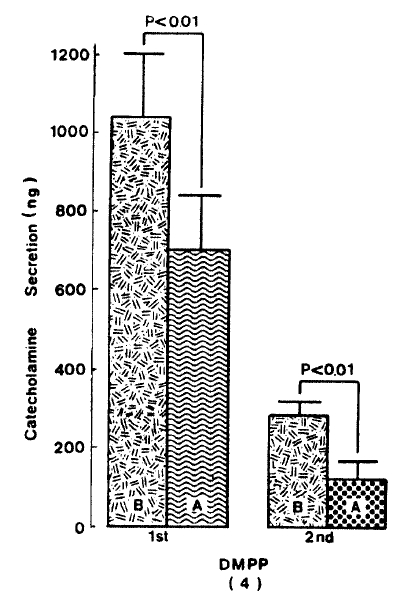
Since it has been found that arachidonic acid dose-dependently stimulates CA release from bovine chromaffin cells in the presence of ouabain and this effect is specific for arachidonic acid15), it was of interest to investigate the effect of arachidonic acid plus ouabain on CA secretion evoked by secretagogues from the isolated perfused rat adrenal glands. When given into an adrenal vein in a volume of 0.05 ml, ACh(5.32×10−3 M) and excess K+ (5.6×10−2 M) produced CA secretion of 600.0±84.3 ng and 373.1±79.6 ng for 4 min, respectively, but in the presence of 10 uM arachidonic acid along with 10−4 M ouabain, which is found to inhibit Na+, K+-activated ATPase and to cause exocytotic secretion of CA29–31) and to enhance the secretory responses to veratridine and carbachol32,33), Ach-stimulated CA secretion was greatly depressed to 447.0±65.3 (p<0.01, n=5) ng for 4 min as compared with its control secretion, as shown in Fig. 4. On the other hand, arachidonic acid plus ouabain treatment did not affect CA secretion by excess K+ and excess K+-evoked CA release amounted to 340.0±54.7 (ns, n=8) ng for 4 min as compared to its control response (Fig. 4).

Effect of arachidonic acid in the presence of ouabain on ACh-and excess K+-evoked CA release. Secretogagues were induced before and after pre-loading with 10 uM arachidonic acid plus 100 uM ouabain for 20 min, respectively. Other legends and methods are the same as in Fig. 1. ns: statistically non-significance. AA: arachidonic acid.
As depicted in figure 5, DMPP (10−4 M)-evoked CA release in the presence of 10−5 M arachidonic acid plus 10−4 M ouabain was significantly inhibited to 555.0±44.8 (0−4 min, p<0.01)ng and 198.7±12.3(4−8 min, p<0.01)ng from 8 rat adrenal glands, respectively, as compared with their corresponding control releases of 840±52.2 (0−4 min) ng and 255.0±6.9 (4−8 min) ng.
After perfusion with arachidonic acid (10−5 M), in the presence of 10−4 M ouabain for 20 min, CA release of muscarine (10−4 M) infused into an adrenalvein for 5 min was prominently attenuated to 267.6±35.4(0−5 min, p<0.01)ng and 202.0±9.2(5−10 min, p<0.01)ng from 5 rats, respectively, as compared with their corresponding control secretions of 446.4±13.8 (0−5 min)ng and 257.7±15.1(5−10 min)ng as shown in Fig. 6.
3. Influence of Arachidonic Acid Plus Indomethacin of CA Release Evoked by ACh, Excess K+, DMPP and Muscarine
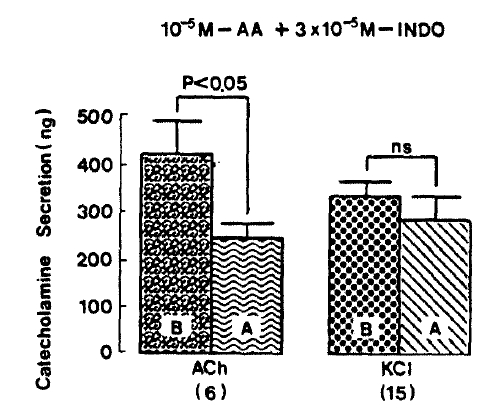
Since it has been shown that indomethacin, an inhibitor of cyclooxygenase, fails to inhibit the stimulatory effect of CA secretion evoked by arachidonic acid in digitonin-permeabilized bovine adrenal medullary cells13) and, it as well as aspirin, has no effect on the release evoked by carbachol and high K+25), it was particularly intersting to test the effect of arachidonic acid plus indomethacin on CA secretion evoked by various secretagogues. As illustrated in Fig. 8, ACh (5.32×10−3 M)- and excess K+ (5.6×10−2 M)-evoked CA secretions before preloading with arachidonic acid plus indomethacin amounted to 420.0±73.4 ng and 337.0±27.5 ng for 4 min, respectively, However, following the pre-perfusion with arachidonic acid(10−5 M) along with indomethacin(3×10−5 M), ACh-induced CA release was significantly reduced to 247.8±24.9 (p<0.05)ng for 4 min from 6 rat adrenal glands, as compared with its control release, while excess K+-induced CA release was 289.3±46.3 ng for 4 min from 15 glands. There was no significant change in excess K+-evoked CA secretion, as compared to tis corresponding control response as shown in Fig. 7.
In the presence of arachidonic acid plus indomethacin, DMPP(10−4 M)-evoked CA secretion was markedly attenuated to 393.8±86.3 (0–4 min, p<0.01, n=8)ng for 4 min, respectively as compared with their corresponding control secretions of 937.5±151.2(0–4 min)ng and 262.5±41.2(4–8 min) ns as shown in Fig. 8.
Muscarine (10−4 M)-evoked release after preloading with arachidonic acid (10−5 M), plus indomethacin (3×10−5 M) for 20 min, was prominently decreased to 94.0±25.0 (0–5 min, p<0.01)ng and 84.5±21.5 (5–10 min, p<0.01)ng free 8 rat adrenal glands, as compared with their corresponding control releases of 220.5±36.3(0–5 min)ng and 122.0±29.4 (5–10 min)ng, respectively. Fig. 9 represents the inhibitory effects of arachidonic acid in the presence of indomethacin on muscarine-evoked CA release
4. Influence of Prostaglandin F2 Alpha on CA Secretion Evoked by DMPP and Excess K+
Since prostaglandin (PG) F2 alpha and PGD2 are found to enhance the basal CA release more than did PGE226), it is exciting to examine the effect of PGF2 alpha on CA secretion evoked by nicotinic receptor stimulation and membrane depolarization. In the present work, when PGF2 alpha was pre-loaded into an adrenal vein for 20 min, excess K+(5.6×10−2 M)-evoked CA secretion, in the pesence of PGF2 alpha, was 341.3±45.2(ns, n=4) ng for 4 min as compared to its control release of 375.0±35.2(n=4)ng/4 min as shown in Fig. 10. Under the effect of PGF2 alpha, DMPP (10−4 M)-evoked CA secretion was greatly inhibited to 705.0±133.5(0–4 min, p<0.01)ng and 127.5±38.0(4–8 min)ng, respectively. Fig. 11 shows the inhibitory effects of PGF2 alpha on DMPP-evoked CA release from the isolated rat adrenal gland.

Effect of prostaglandon F2 alpha on excess K+-evoked CA release from perfused rat adrenal glands. Excess KCl (5.6×10−2M) was injected into an adrenal vein before and after preperfusion with 100 nM pro-staglandin F2 alpha for 20 min, respcetively. Other legends and methods are as in Fig. 1. PGF2 alpha: prostaglandin F2 alpha.
DISCUSSION
The present investigation demonstrates that arachidonic acid causes the inhibitory effect of CA release evoked by cholinergic receptor-mediated stimulation but not by membrane depolarization in the isolated perfused rat adrenal medulla, and that it also plays a modulatory role in regulating CA secretion. However, in previous studies, Nishibe and his colleagues (1983)27) found that addition of arachidonic acid to intact bovine chromaffin cells induces CA secretion. It has been also shown that chromaffin cells from bovine adrenal medulla can be stimulated by external arachidonic acid at low concentrations (<5 uM) to about 50% of maximal release14).
In contrast with previous reports, the present experimental results showed that pre-loading with arachidonic acid significantly attenuated CA secretion evoked by ACh, DMPP and muscarine, but not by excess K+. From these findings, it is felt that there may be a species difference between animal models. However, it has been reported that concentrations of arachidonic acid, exceeding 50 uM, usually exhibit cytotoxic effects in human neutrophils28). In terms of this fact, it could not be ruled out that concentration of arachidonic acid used in the present experiment may produce cytoxic effect in the rat adrenomedullary chromaffin cells. Furthermore, the present work also showed that arachidonic acid, in the presence of oubain effect, exhibited the marked inhibition of CA secretion evoked by cholinergic secretagogues, but not by membrane depolarization. However, Negishi, Ito and Hayaishi (1990)15) have reported that arachidonic acid dose-dependently stimulates CA release from cultured bovine adrenal chromaffin cells in the presence of ouabain and this effect is specific for arachidonic acid. Moreover, several investigators have found that the inhibition of Na+, K+-ATPase by ouabain or by the omission of K+ from the incubation medium by itself causes the exocytotic secretion of CA29–31) and enhances the secretory responses to veratridine and carbachol32,33). It has been also demostrated, recently, that PGE2 can cause a drastic increase in CA release from cultured bvine adrenal chromaffin cells in the presence of ouabain, an inhibitor of Na+, K+-ATPase, whereby PGE2 stimulates phosphoinositide metabolism accompanied by an increase in intracellular Ca++34). Tanaka et al (1990)35), have reported that PGE2 activates the Na+, H+-antiport by stimulating phosphoinositide metabolism and that the increase in intracellular Na+ by both inhibition of Na+, K+-ATPase and activation of Na+, H+-antiport may lead to the redistribution of Ca++, which is the initial trigger of CA release. Regardless of these previous results, in the present study, the perfusion of ouabain (100 uM) itself for 20 min, as well as coadministration along with arachidonic acid, did not affect basal release of CA in the rat adrenal gland. Arachidonic acid in the presence of indomethacin, which is known to be an inhibitor of cyclooxygenase36), also inhibited CA release evoked by ACh, DMPP and muscarine, while it did not affect the secretory response of CA induced by excess K+. Thus, it is thought that the inhitory effect by arachidonic acid of chloinergic receptor stimulation-induced CA release is not associated with cyclooxygenase activation or induction. In support of this idea, in a variety of tissues, it has been illustrated that arachidonic acid is metabolized to form prostaglandins and hydroxy fatty acids through the cyclooxygenase and lipoxygenase pathways37). Koda and his associates (1989)13) showed that indomethacin, an inhibitor of cyclooxygenase and nordihy-droguaiaretic acid, an inhibitor of lipoxygenase, failed to inhibit the stimulatory effect of arachidonic acid an Ca++-induced CA secretion in digitonin permeabilized bovine adrenal medullary cells. However, inhibition of prostaglandin biosynthesis by indomethacin potentiated CA release from cat adrenal gland38), whereas indomethacin failed to facilitate epinephrine efflux from a perfused rabbit adrenal gland39). The reason for the discrepancies among different authors’ results is not known, but may be due to differences in experimental design or animal species used.
In the present investigation, pretreatment with prostaglandin F2 alpha, one of arachidonic acid metabolites, markedly depressed DMPP-evoked CA secretion while it did not modify the secretory effect of CA induced by excess K+. These effects were very similar to those evoked by arachidonic acid alone. In support of this finding, PGE2, one of arachidonic acid metabolites, is present in varous mammalian tissues and exerts diverse biological effects in-vivo and in-vitro40). For instance, PGE2 is known to inhibit the release of norepinephrine from sympathetic nerve terminals in response to Ach41–42) and also to inhibit basal and ACh-induced CA secretion from bovine and rat adrenal glands43–45). Kilpatrick et al. (1981)46) found that PGE1 slightly inhibited nicotine-induced CA release by using cultured bovine chromaffin cells. Because ACh stimulates the formation of prostaglandins, Hedqvist (1977)47) proposed that PGE2 could act as a negative feedback inhibitor of norepinephrine release. Taken together with those previous results, it is considered that PGF2 alpha in the present experiments inhibits CA secretory effect evoked by nicotinic receptor stimulation, although it was not established whether the effect of PGF2 alpha is mediated by a specific receptor located on the rat adrenomedullary chromaffin cells. On the contrary, in the anesthetized dog, high concentrations of PGE2 were reported to stimulate the basal release of CA and also to act synergistically with ACh in evoking CA secretion48). Moreover, it has also been demonstrated that PGE2 drastically enhances CA release from bovine adrenal chromaffin cells in the presence of ouabain through the PGE receptor33,35). PGE2 was also reported recently to stimulate basal and evoked release of CA26,49–55), implying that PGE2 may serve as a stimulant. However, PGF2 alpha and PGD2 enhanced the basal CA release more than did PGE226). On the other hand, in the present study, PGF2 alpha itself did not affect basal release of CA but significantly inhibited nicotinic receptor stimulation-induced CA secretion as the experimental results in previous studies43–45).
Anyway, arachidonic acid, as well as PGF2 alpha, appear to inhibit nicotinic receptor stimulation-evoked CA secretion but did not membrane depolarization-induced secretion from the isolated perfused rat adrenal medulla, and also play a modulatory role in regulating CA release.