Impairment of Endothelium-Dependent Relaxation in Chronic Two-Kidney, One Clip Hypertensive Rats
Article information
Abstract
Objectives
Hypertension is commonly associated with an endothelial dysfunction that may contribute to the rise in blood pressure. Little information has been available so far on the role of endothelium-derived nitric oxide(EDNO) in renin-dependent, 2-kidney, 1 clip(2KIC) hypertension. The present study was aimed to determine a role for EDNO in the development and maintenance of 2KIC hypertension.
Methods
The effects of blocking synthesis or supplementation with precursor of EDNO on the development of hypertension were determined in 2KIC rats. Vascular responses to acetyl-choline, nitroprusside, atrial natriuretic peptide and nifedipine were examined in 7-and 12-week hypertensive 2KIC rats.
Results
NG-nitro-L-arginine-methyl ester caused a sustained increase of blood pressure in normal rats, while it was only partially associated with a more pronounced increase of blood pressure in the developmental phase of hypertension in 2KIC rats. In 7-week and 12-week hypertensive rats, phenylephrine-induced contraction of the isolated thoracic aortic rings was more sensitive compared with control. Their acetylcholine-induced relaxation was attenuated while the responses to nitroprusside or atrial natriuretic peptide were unaltered. Although their blood pressure did not differ between 7-week and 12-week hypertensive groups, the attenuation in the acetylcholine-induced relaxation was more prominent in the latter with a longer duration of hypertension. Indomethacin did not affect the attenuated relaxation to acetylcholine. The relaxation response to nifedipine was more pronounced in 2KIC rats.
Conclusion
These results indicate that EDNO has little influence of the 2KIC hypertension, at least during its developmental phase, which is associated with an activated renin-angiotensin system. The chronic stage of 2KIC hypertension, however, is associated with an endothelial dysfunction which may contribute to the enhanced vasoconstriction and sustained high blood pressure.
INTRODUCTION
It is now known that the vascular endothelium plays an important role in modulating vascular tone through production of factors causing either relaxation or contraction of the underlying smooth muscle layer1). Systemic blockade of endothelium-derived nitric oxide(EDNO) synthesis results in increases of vascular resistance and blood pressure in anesthetized rats2). Moreover, hypertension is commonly associated with an endothelial dysfunction that may contribute to the sustained rise in blood pressure3–6).
In animal models of hypertension including 2-kidney, 1 clip(2KIC) and spontaneous hypertension, the endothelium-dependent vasodilation is found to be impaired and restored by reversing the hypertension with antihypertensive therapy3–6). Similar abnormalities have been reported in humans with essential hypertension7–8).
With the increasing knowledge on endothelium-dependent vascular regulatory mechanisms, interactions between endothelial mediators and other hormonal systems become important. Although an interaction between EDNO and renin has been extensively documented, the role for NO in renin secretion is still controversial. Vidal et al.9) and Beierwaltes et al.10) have found that EDNO inhibits renin release from renal cortical cells in vitro. A stimulatory effect on renin secretion was also observe11,12).
The increased blood pressure may result in an increasing shear stress and stimulus for the release of EDNO in the vasculature13). An increased EDNO production may then counterbalance the influence of the elevated circulating constrictors. Sigmon et al14) have indeed found that EDNO maintains renal perfusion of the non-clipped kidney in 2KIC rats. Little information has been available so far, however, on the pathophysiological role of EDNO in renin-dependent 2KIC hypertension.
The present study was aimed to investigate to what extent and how EDNO is implicated in 2KIC hypertension. The effects of blocking synthesis or supplementation with precursor of EDNO on the early development of high blood pressure were determined in 2KIC rats. In addition, vasoconstrictor and dilator responses of the isolated vasculature from chronically hypertensive 2KIC rats were examined.
METHODS
Development of 2KIC Hypertension
Male Sprague-Dawley rats(150–200g) were made hypertensive by constricting the left renal artery with a silver clip having an internal gap of 0.2mm under ketamine anesthesia. The contralateral kidney was left untouched. The rats were then divided into three groups. The one group was supplemented with NG-nitro-L-arginine-methyl ester(L-NAME, 5mg/100 ml), another with L-arginine(400mg/100 ml) in the drinking water, and the other was control supplied with normal tap water. Additional three groups without clipping were also provided, and allocated as in 2KIC rats, i.e., L-NAME, L-arginine, and control groups.
Systolic blood pressure(SBP) was indirectly measured by means of a tail cuff method in conscious, prewarmed(37°C for 10 min) rats. The basal blood pressure was obtained as an average of the values taken 3 consecutive days before clipping the renal artery. Following the clipping, SBP was measured on Days, 7, 14, 21 and 28. On Day 28 upon measuring SBP, the animals were decapitated and the trunk blood was collected for measurement of plasma renin concentration(PRC) by radioimmunoassay.
Isolated Vascular Preparations in Chronic 2KIC Hypertension
In another series of experiment, 2KIC rats were kept on a normal diet for 3, 7 or 12 weeks. On the experimental day, their blood pressure was measured directly from a femoral artery under pentobarbital anesthesia. Thoracic aortae were then taken and prepared into the rings 5-mm long each in ice-cold saline. Each ring was suspended in a tissue bath containing physiologic salt solution at 37 ± 0.5°C, continuously bubbled with 95% O2-5% CO2. The one end of the ring was fixed to the bottom of the bath and the other attached to a force-displacement transducer(Grass FTO3) to record its isometric tension. Baseline load placed on the ring was 2.0g. The composition(in mmol/L) of the physiologic salt solution used was NaCl 112, KCl 5, NaHCO3 25, KH2PO4 1.0, MgSO4 1.2, CaCl2 2.5, and glucose 11.5.
A cumulative dose-contraction curve to phenylephrine was obtained. Dose-relaxation curves to acetylcholine, nitroprusside, atrial natriuretic peptide or nifedipine were obtained in the aortic rings precontracted with EC so dose of phyenylephrine(3.5×10−6 mol/L). Relaxation was calculated as percent reductions from the phenylephrine-induced maximum contraction.
Drugs used were phenylephrine(Sigma), acetylcholine chloride(Sigma), L-NAME(Sigma), L-arginine(Choongwae), nitroprusside(Roche), atrial natriuretic peptide(atriopeptin III, Sigma) and nifedipine(Sigma).
Statistics
Results are expressed as means ±SEM. SBP was compared by two-way ANOVA between the groups. PRC was compared between the groups using Student t-test.
RESULTS
Effects of Blocking Nitric Oxide Synthesis or Supplementation with its Precursor on the Development of Hypertension
Fig. 1 shows the development of hypertension in 2KIC rats. Basal SBP in control, L-NAME-and L-arginine-supplemented groups did not significantly differ. In all three groups, SBP progressively increased during the experimental period. L-NAME did not affect the blood pressure, except at Day 21 when SBP was significantly higher compared with the control. L-arginine had no significant effect on SBP. PRC measured at Day 28 were 30.7 ± 3.2 in control(n=6), 20.6 ± 3.2 in L-NAME(n = 7), and 58.6 ± 16.9ngAI/mL/h in L-arginine groups(n = 6). It was significantly lower in L-NAME group and higher in L-arginine group than in control.
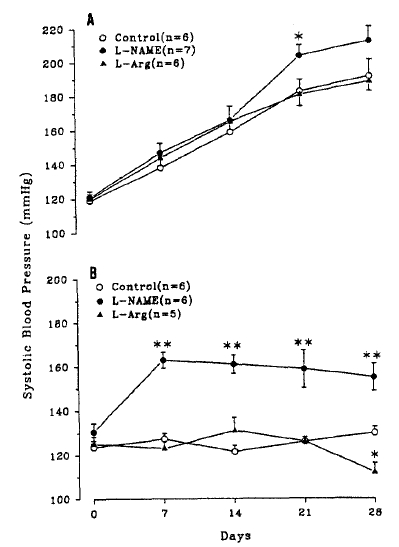
Effects of L-NAME or L-arginine ingestion on systolic blood pressure in 2KIC(A) and normal rats(B). n=number of rats. *p < 0.05, **p < 0.01;compared with control.
Fig. 1 also shows the blood pressure in normal rats. L-NAME significantly increased SBP, while L-arginine was without effect. In these rats, PRC did not differ among the groups, i.e., 16.7 ± 1.9 in control(n = 6), 17.9 ± 2.1 in L-NAME(n = 6), and 18.0 ± 1.3 ngAl/mL/h in L-arginine group(n=5).
Vascular Responses in Chronically Hypertensive Rats
In chronically hypertensive rats, the mean arterial pressures measured directly under anesthesia were 147 ±9 in 3-week(n=8), 179 ± 10 in 7-week(n=8) and 178 ± 6 mmHg in 12-week hypertensive rats(n=8). They were significantly higher compared with the control 103±3 mmHg(n=8).
Phenylephrine contracted aortic rings in a dose-dependent manner, in which 2KIC rats showed more sensitive response than the control(Fig. 2). Relaxation response to acetylcholine of the phenylephrine(3.5×10−6 mol/L) – precontracted aortic rings was attenuated in 2KIC rats(Fig. 3), which was not affected in the presence of indomethacin(10−6 mol/L). Hypertension of a longer duration was associated with a more prominent decrease of acetylcholine-mediated response(12-week > 7-week). The relaxation in response to either nitroprusside or atrial natriuretic peptide was not altered(Fig. 4). Three-week hypertensive rats showed neither an enhanced contraction to phenylephrine nor an attenuated relaxation to acetylcholine.

Cumulative dose-contraction curves in response to phenylephrine of isolated thoracic aortic rings from normotensive(control), 7-week(7W), and 12-week(12W) hypertensive rats. Each point represents mean ±SEM from 18–24 experiments. Contraction is expressed as a percentage of the maximum response.

Dose-relaxation curves in response to acetycholine of isolated thoracic aortic rings from control, 7-week, and 12-week hypertensive rats. Each point represents mean ±SEM from 16–19 experiments. Relaxation is expressed as a percentage change from the maximum contraction attained by phenylephrine(3.5 × 10−6 mol/L).

Dose-relaxation curves in response to nitroprusside and atrial natriuretic peptide of isolated thoracic aortic rings from control and 12-week hypertensive rats. Each point represents mean ±SEM from 13–26 experiments.
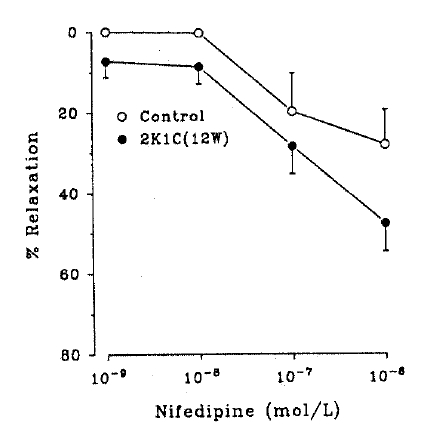
The relaxation response to nifedipine was more sensitive in the hypertensive aortic rings(Fig. 5).
DISCUSSION
Oral ingestion of L-NAME caused a sustained increase of blood pressure in normal rats, while it was without effect except a higher value only at Day 21 in 2KIC rats. This finding confirms a major role of EDNO in the long-term regulation of arterial pressure and suggests that EDNO does not strongly affect the renin-dependent 2KIC hypertension at least during its developmental phase. It may also indicate that the EDNO blockade and enhanced angiotensin n activity have the common pressor mechanism, possibly on the vasculature. The already powerful vasoconstrictor effect of angiotensin II may mask an effect of L-NAME in 2KIC rats, or vice versa. In fact, previous investigators have shown that L-NAME increases vascular reststance in renal, mesenteric and hindquarter vascular beds15,l6). L-NAME may produce a vasoconstrictor type of hypertension.
In contrast, Hashikawa et al17). reported that intravenous administration of L-arginine decreased arterial pressure in association with elevated cardiac ouput and reduced total peripheral resistance in patients with primary and secondary hypertension. They suggested that exogenous L-arginine could produce a vasodilator effect via stimulating synthesis and release of EDNO in hypertension. In our study, ingestion of L-arginine did not modify blood pressure in 2KIC rats, whereas it significantly lowered it after 28 days of ingestion in normal rats. This finding suggests, among others, that substrate availability is not a critical factor in determining the effect of EDNO on blood pressure in 2KIC rats.
Under various experimental conditions, the effect of EDNO on renin secretion is not unequivocal. There are observations suggesting an inhibitory9,10,18) as well as a stimulatory effect11,12). The effects of EDNO blockade on the renin-angiotensin system may be highly complex in vivo. In addition to removal of any direct influence of EDNO an renin secretion, there are factors such as increased renal perfusion pressure to suppress and a stimulatory effect on prostacyclin19), which in turn, to enhance it. Although EDNO blockade did not either potentiate or attenuate the hypertension in 2KIC rats, PRC was higher in the L-arginine and lower in L-NAME group compared with the control. Therefore, the different PRC may not be attributed to blood pressures but to direct stimulatory or inhibitory effect of L-NAME or L-arginine on the renin release.
On the other hand, chronic hypertension was associated with reduced endothelium-dependent relaxation of the isolated thoracic aortic rings, with the response to either nitroprusside or atrial natriuretic peptide being unaltered. The attenuation in acetylcholine-mediated response was more prominent in the 12-week hypertensive than in the 7-week hypertensive despite the comparable blood pressure between these groups. This is in line with a previous observation that vascular responses to vasodilator agents varied according to duration of the hypertension20). Thus, the impairment appears to develop secondarily in response to prolonged exposure of the endothelium to the high blood pressure. The speculation is further strengthened by the finding that the magnitude of acetylcholine-induced relaxation was comparable between the aortic rings from 3-week hypertensive and control rats. The endothelial dysfunction, however, may culminate in an enhanced pressor response(shown by a more sensitive vasoconstriction response to phenylephrine) to maintain the high blood pressure.
The abnormal endothelium-dependent relaxation has been attributed to an alteration in the balance between the release of endothelium-derived relaxing and contracting factors. An increased release of a contracting factor, which is inhibited by indomethacin, has been suggested in spontaneously hypertensive rats21,22). Indomethacin has also been found to increase the maximum response to acetylcholine in 2KIC rats16). The attenuated relaxation was not affected by indomethacin in the present study, however, suggesting that the endothelial dysfunction is not due to a cyclooxygenase pathway product. The discrepancy between this and the previos studies are associated with three different conditions, i.e., the duration of hypertension(12 versus 10 weeks), the vascular species used(thoracic aorta versus mesenteric resistance arteries), and the dosage of indomethacin(10−6 versus 10−5 mol/L). While the former two issues cannot be explained, we believe a higher dose of indomethacin undesirable since it could affect the relaxation or contraction of the vascular preparations.
Furthermore, the vasorelaxtion response to nifedipine was more sensitive in the hypertensives. This is in agreement with the previous finding in which the vasodilating effect of nifedipine is stronger in hypertensive than in normotensive individuals23). These results explain why nifedipine produces a greater reduction of blood pressure in patients with hypertension compared with normotensive individuals24,25) Taken together, it is likely that the action of nifedipine is more powerful if the blood vessels are already in a high tonus(vasoconstricted). Alterations in permeability various ionic species of the vasculature in hypertension are also worth pursuing.
In summary, our study demonstrated that EDNO has little influence on the development of 2KIC hypertension in which renin-angiotensin system has already been in its activated state. The different PRC between the groups supplemented with L-NAME or L-arginine may not be attributed to a difference in blood pressure, but to direct influence of either drug on EDNO. The endothelial dysfunction in the late stage of 2KIC hypertension may contribute to the enhanced vasoconstriction and sustained high blood pressure.