Overweight and Effect of Hormone Replacement Therapy on Lipid Profiles in Postmenopausal Women
Article information
Abstract
Background
Many experimental and observational studies have suggested that hormone replacement therapy (HRT) in postmenopausal women is cardioprotective. However, the results of randomized controlled trials have been discouraging. We attempted to evaluate the influence of overweight, a frequent risk factor for coronary artery disease, on the lipid-modifying effects of HRT.
Methods
A total of 345 postmenopausal women were divided into 2 groups according to body mass index (BMI): the control group; BMI<25 Kg/m2 (n=248) and the overweight group; BMI≥25 Kg/m2 (n=97). All women received either 0.625 mg conjugated equine estrogen (CEE)(n=139), CEE plus 5 mg medroxyprogesterone acetate (MPA)(n=97) or CEE plus 10 mg MPA (n=109). Lipid profiles were measured before and 12 months after HRT.
Results
In both the control and overweight groups, HRT reduced low density lipoprotein cholesterol (LDL-C) (p=0.000 and p=0.000 respectively) and lipoprotein (a) [Lp(a)] levels (p=0.000 and p=0.000 respectively) and raised high density lipoprotein cholesterol (HDL-C) levels (p=0.000 and p=0.002 respectively). However, the elevation of the HDL-C level was higher in the control group than in overweight group (17.5% vs. 10.4%, p=0.015), and this was significant after adjusting for changes in body weights (p=0.016). There were no differences in the reduction of LDL-C (p=0.20) and Lp(a) (p=0.09) levels between the two groups.
Conclusion
HRT had less favorable effects on HDL-C levels in overweight postmenopausal women than in women with normal body weight. This finding may be partially associated with no cardioprotective effect of HRT in postmenopausal patients at a high risk due to multiple risk factors including obesity.
INTRODUCTION
A lot of experimental investigations and population-based observational studies have reported that estrogen replacement therapy (ERT) in postmenopausal women is cardioprotective1-4). Proposed mechanisms for this process are the modification of lipid profiles, improvement of endothelium dependent vasoreactivity, antioxidant effects and so on1, 2, 5-8). The effect of progesterone is still being debated. Although many reports have shown that progesterone may negate the beneficial effects of estrogen1, 8-11), several clinical studies have suggested that estrogen/progesterone combination therapy may be at least as effective as ERT12, 13).
The first randomized controlled trial showed that combination therapy of estrogen and progesterone did not reduce the overall rate of coronary heart events, and increased the rate of adverse effects of drugs in postmenopausal women with coronary artery disease14). In the first year of follow-up, a significant 52% increase in cardiovascular events was observed15). After an average of 4.1 years of follow-up, this increase became negligible. Several hypotheses have been proposed to explain the discordance between observational and randomized trials. These include an inadequate duration of follow-up, the adverse effects of progesterone, the advanced ages of the study subjects, and so on15, 16). Recently, a randomized primary prevention trial was stopped early due to small but consistent adverse cardiovascular effects of estrogen and progesterone therapy in postmenopausal women17).
Obesity is one of the classic risk factors for atherosclerosis18, 19). It has pleiotrophic actions, such as elevations of blood pressure and atherogenic lipoproteins, and insulin resistance resulting in metabolic syndrome in genetically susceptible individuals20). Adult Treatment Panel III guideline of the National Cholesterol Education Program recently emphasized the importance of metabolic syndrome and its management by weight control and by other non-drug methods21).
In this study, we attempted to evaluate whether overweight, a frequent risk factor for coronary artery disease, influences the effects of HRT on lipid profiles in postmenopausal women or not.
MATERIALS AND METHODS
Study population and design
The subjects of this study were part of a previous case-controlled study to evaluate the effects of hormone replacement therapy on lipid and lipoprotein levels according to the duration of medication and the androgenic effects of progesterone9). A total of 345 postmenopausal women who had been amenorrheic for 1 year or longer without evidence of gynecological disorders and/or who had a follicle stimulating hormone level (FSH) greater than 20 mIU/L were enrolled. Subjects with diseases which influence lipid levels, such as diabetes mellitus, chronic liver diseases, infectious diseases or other endocrine diseases, were excluded. None had received hormonal preparations or medications which changed lipid levels before or during the study. The study subjects were divided into two groups according to body mass index (BMI): the control group; BMI < 25 Kg/m2 (n=248) and the overweight group; BMI ≥ 25 Kg/m2 (n=97).
All women received either 0.625 mg conjugated equine estrogen (CEE) (n=139), 0.625 mg CEE plus 5 mg medroxyprogesterone acetate (MPA) (n=97) or 0.625 mg CEE plus 10 mg MPA (n=109). Medication was prescribed cyclically every 30 days. CEE was administered from the 1st to the 25th days, and no medication was given from the 26th to the 30th days. MPA was added from the 16th to the 25th days, when indicated.
Measurements
After overnight fasting, blood samples were obtained. Total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), very low density lipoprotein cholesterol (VLDL-C), triglyceride, and lipoprotein (a)[Lp(a)] levels were measured before and 12 months after medication.
Total cholesterol and triglyceride concentrations were determined by the enzymatic method, using an automatic analyzer (Model 7150, Hitachi, Naka, Japan). The concentrations of HDL-C, LDL-C and VLDL-C were determined by electrophoretic methods using the HDL Cholesterol Supply Kit (Helena Laboratory, Beaumont, TX). The lipoproteins were separated according to their electrophoretic mobility on cellulose acetate in a tris-barbital buffer, pH 8.8 and fractions were visualized with production of quinoneimine by the enzymatic method. The relative percentage of each fraction was obtained by scanning in a densitometer equipped with a 500 or 505 nm filter. The HDL-C, LDL-C and VLDL-C concentrations were calculated by multiplying each ratio by the total cholesterol concentration.
The concentration of Lp(a) was determined by 2-site immunoradiometric assay using a commercial radioimmunoassay kit (Pharmacia, Uppsala, Sweden) as previously described9). FSH level was measured by immunoradiometric assay using a radioimmunoassay kit (Serono Diagnostic, Rome, Italy).
Statistics
Data were expressed as mean±SD. The data were stored on an IBM computer using dBASE III plus (Borland Co, Scotts Valley, CA). Statistical analysis was performed using the Social Package for Social Science (SPSS Inc., Chicago, IL). For the analysis of Lp(a), VLDL-C, and triglyceride levels, the Mann-Whitney U test was used to evaluate differences between groups, and the Wilcoxon signed-rank test was used to compare the concentrations before and after medication. For other variables, the Student t-test was used to evaluate the differences between groups, and the paired t-test was used to compare the concentrations before and after medication. The distribution of different combinations of HRT was analyzed by the χ2-test. The adjustment of lipid profiles by changes in body weights during the follow-up period was done using the linear regression method. Two-tailed values of p<0.05 were considered to be statistically significant.
RESULTS
Baseline characteristics of subjects
Table 1 shows the baseline clinical and biochemical characteristics of the two groups. The control group was taller than the overweight group (p=0.001). Fasting blood glucose levels were similar between the two groups (p=0.42). There were no differences in the prescription of hormones between the two groups (p=0.16).
As expected, overweight postmenopausal women had higher levels of total cholesterol (p=0.042), LDL-C (p=0.025), VLDL-C (p=0.000) and triglyceride (p=0.000) than did normal weight women (Table 2). There were no differences in HDL-C levels (p=0.18) between the two groups. Lp(a) level was lower in the overweight group than in the control group with borderline significance (p=0.078).
Effect of HRT in the control and overweight groups
Body weight remained unchanged in the control group (p=0.50) and decreased in the overweight group (p=0.001) (Table 2). HRT for 12 months lowered the levels of LDL-C (p=0.000 in both groups) and Lp(a) (p=0.000 in both groups), and elevated HDL-C levels (p=0.000 and p=0.002 in the control and overweight groups respectively). VLDL-C levels increased in the control group, with borderline significance (p=0.074), and decreased in the overweight group (p=0.005). As a result of these changes, total cholesterol level was not changed in the control group (p=0.42) and decreased in the overweight group (p=0.000). Triglyceride levels increased in the control group (p=0.019) and decreased in the overweight group (p=0.029).
After HRT for 12 months, all lipid profiles except HDL-C were similar between the two groups. HDL-C levels were lower in the overweight group than in the control group (p=0.000).
Differences in the change of lipid profiles after HRT according to obesity
The elevation of HDL-C levels was significantly more pronounced in the control group than in the overweight group (0.31±0.41 vs. 0.17±0.55 mmol/L, p=0.015) (Table 3, Figure 1). This difference remained significant even after adjusting for changes in body weights (p=0.016). There were no significant differences in decreases in LDL-C levels between the two groups (0.36±0.56 vs. 0.46±0.68 mmol/L, p=0.20). The reductions in LDL-C levels were also insignificant after adjusting for changes in body weight (p=0.25). Differences in the changes in total cholesterol, triglyceride and VLDL-C levels were significant (p=0.001, p=0.002 and p=0.001 respectively). Overweight had a borderline effect on the decreases of Lp(a) levels (11.8±15.7 vs. 9.3±15.4 mg/dL, p=0.090).
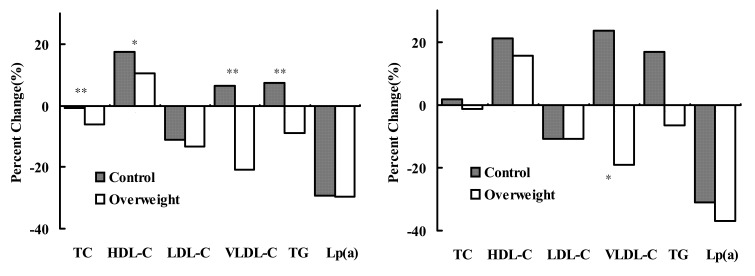
Percent changes in lipid profiles after hormone replacement therapy according to obesity in the total cases (left), and in the subgroup receiving estrogen only (right). TC: total cholesterol, HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; VLDL-C, very low density lipoprotein-cholesterol, Lp(a):lipoprotein(a). *: p<0.05, **: p<0.005.
DISCUSSION
This post-hoc analysis of the case-controlled study showed that HRT had less favorable effects on HDL-C levels in overweight postmenopausal women than in women with normal body weight. To our knowledge, this is the first report that directly evaluated the influence of overweight on the lipid modifying effects of HRT.
In this study, HDL-C concentrations were measured by an electrophoretic method which results in higher values than are generated by precipitation methods. Therefore, HDL-C levels in this study were higher than the values reported in most studies. The validation of this method and the comparison with classic methods were reported previously9).
A great many in vitro experimental and animal investigations have shown that estrogen may delay atherosclerosis1, 2, 22). A lot of clinical observational studies have also reported that ERT reduces the incidence of coronary artery disease by about 30~50%1-4). Proposed mechanisms for this cardioprotective effect of estrogen are 1) the modification of lipid profiles, such as the reduction of LDL-C and Lp(a) levels and the elevation of HDL-C levels 2) improvement of endothelium dependent vasoreactivity through NO related processes 3) antioxidant effects, such as the inhibition of LDL oxidation and 4) others, including antithrombotic and fibrinolytic effects, changes in the smooth muscle cells and matrix, and angiogenesis1, 2, 5-8). There is a controversy regarding to the effects of progesterone. Although several clinical studies have suggested that estrogen/progesterone therapy is at least as effective as estrogen alone12, 13), many reports have shown that progesterone may negate the effects of estrogen1, 8-11). In this study, the effects of HRT on lipid profiles were consistent with the findings of previous reports, as LDL-C and Lp(a) levels decreased and HDL-C level increased regardless of body mass index.
A few epidemiological studies have been conducted to observe the effect of estrogen on the secondary prevention of coronary artery diseases4, 23-25). This effect seemed to be more effective than primary prevention effect. However, these studies had inadequate designs and small numbers of cases to have statistical power. Recently, the first large-scale randomized secondary prevention trial, the Heart and Estrogen/progestin Replacement Study (HERS), was reported14). After an average of 4.1 years of follow-up, there were no differences in the incidence of nonfatal myocardial infarction and coronary heart disease death between estrogen/progesterone and placebo groups in postmenopausal women with coronary artery disease. Adverse events, such as venous thromboembolism and gallbladder disease, were more frequent in the HRT group than in the placebo group. Following randomized reports, such as the Estrogen Replacement and Atherosclerosis (ERA) study with angiographic end point26) and the study regarding the progression of carotid intima-media thickness27), supported the results of the HERS study.
Several explanations have been proposed to explain a lack of secondary prevention effects of HRT. These include inadequate duration of follow-up, the adverse effects of progesterone, advanced age of the subject women and so on15, 16). In observational studies, the HRT group tended to be relatively healthier, more educated, and of higher economic class than non-user group28, 29). Although these differences have been statistically adjusted, selection bias may remain.
Recently, another randomized controlled study, the Women Health Initiative (WHI) study, reported that HRT might be harmful to the cardiovascular system of healthy postmenopausal women. Combined therapy of 0.625 mg of CEE and 2.5 mg of MPA increased incidences of cardiovascular disease and breast cancer, and the study was stopped early.
Progesterone administered with estrogen may abolish the cardioprotective effects of estrogen in these studies. In the WHI study, an attempt to evaluate the effects of estrogen in women who have had hysterectomy is being continued because the balance of overall risks and benefits remains uncertain at an average follow-up of 5.2 years17). However, in the ERA study26), the estrogen-only group also did not affect the progression of coronary artery lesions. In this study, the subgroup receiving only estrogen had similar changes in lipid profiles with the total population according to obesity, although these were statistically insignificant due to the small number of cases (Figure 1).
Obesity is strongly predictive of higher incidences of coronary artery disease, stroke, and mortality17, 18). Obese people may have other risk factors including high blood pressure, dyslipidemia, and insulin resistance19). Obesity itself without these risk factors is at an increased risk of cardiovascular disease. A large proportion of patients with coronary artery disease has overweight as one of risk factors. In this study, lipid profiles in overweight women were similar to those observed in previous reports, higher levels of total cholesterol, LDL-C, VLDL-C and triglyceride. In contrast to previous results, there were no differences in HDL-C levels between the control and overweight groups. This finding may be partially explained by the exclusion of patients with diabetes mellitus in subjects. Actually, fasting blood sugar levels were similar between the two groups.
Obese postmenopausal women have higher estrone levels than lean women, because estrone is produced by the aromatization from androgen in peripheral adipose tissue30, 31). The effects of endogenous estrogen on lipid profiles were controversial in men and women32-35). However, endogenous estrogen was associated negatively with LDL-C levels and positively with HDL-C levels in postmenopausal women34, 35). Therefore, HDL-C levels in postmenopausal women may be determined by the complex interaction of endogenous estrogen levels and obesity itself. In one report, weight reduction in obese men increased HDL-C levels and, in contrast, lowered HDL-C levels in obese women36). In this study, basal HDL-C levels were similar between the two groups. This finding might result from the combination of the HDL-C lowering effect of overweight itself17-19) and the HDL-C elevating effect of high estrone levels in overweight women30, 31).
There has been only one cross-sectional study to compared the effects of HRT on lipid profiles between postmenopausal women with and without obesity37). Obese users of HRT had HDL-C levels similar to those of obese never users and overweight former users. However, no case-controlled studies have, to our knowledge, ever been reported previously. In this study, the effects of HRT on HDL-C levels were different between the overweight and control groups. Although HDL-C levels increased in both groups, the degree of elevation was lower in the overweight women than in the women with normal weight (10.4% vs. 17.5%, p=0.015). This difference was significant, even after adjusting for body weight changes during the follow-up period (p=0.16). Changes in LDL-C levels were similar between the two groups.
The effects of exogenous female sex hormone on HDL-C levels might be attenuated in the overweight postmenopausal women by their higher endogenous estrone levels resulting in relatively higher basal HDL-C levels. With the administration of exogenous female sex hormones, the effects of endogenous estrogen might be eliminated and the effects of overweight might become dominant. In this study, the follow-up HDL-C levels were higher in the control group than in the overweight group. However, we could not reach this conclusion because we did not measure the levels of female sex hormones, and could not analyze the relationship between endogenous female sex hormones and lipid profiles. Further study will be needed to confirm this hypothesis.
In men, both LDL-C and HDL-C are strong and independent predictors of coronary artery disease. However, HDL-C plays a more important role in the development of coronary artery disease in women38). HDL-C level was the most powerful predictor of cardiovascular death except for age in the Lipid Research Clinic Follow-up Study38). Total cholesterol significantly increases the risk only at very high levels. LDL-C was a less powerful predictor than HDL-C or even not a significant independent factor in women38). Therefore, the less elevation of HDL-C level in overweight women may attenuate the cardioprotective effects of HRT compared with women with normal body weight. Considering the frequent association of overweight with patients at high risk to develop coronary artery disease, this phenomenon may be partially associated with a lack of cardioprotective effects of HRT in primary and secondary prevention trials.
In this study, total cholesterol level decreased only in the overweight women. However, this decrease was not due to the reduction of LDL-C levels but due to the less elevation of HDL-C levels and greater reduction of VLDL-C levels. Changes in VLDL-C and triglyceride levels were in opposite directions between the two groups, increasing in the control group and decreasing in the overweight group. This finding may be partially explained by the decrease of body weight in the overweight group. Estrogen replacement therapy in postmenopausal women increases VLDL levels resulting in elevations in VLDL-C and triglyceride levels. However, these increases are not due to atherogenic lipoprotein, small dense VLDL, but to large VLDL with low density. The clinical significance of increased VLDL and triglyceride levels with HRT has not yet been established8). Therefore, we do not think that these differences may significantly influence the cardioprotective effects of HRT.
In contrast to other lipids and lipoproteins, Lp(a) levels revealed a trend to be lower in the overweight group than the control group with borderline significance (p=0.078). Lp(a) levels are determined mainly by genetic background, and are not influenced by obesity39). Only few drugs such as niacin and HRT can decrease Lp(a) levels9, 39). So low Lp(a) levels in overweight postmenopausal women might be explained by high estrone levels as described above.
There are several limitations of this study. We retrospectively reanalyzed the data of a previous case-controlled study. This study was performed with a selected population from a single center. Therefore a prospective study including the general population may be needed to confirm the results. We suggested the possibility that endogenous estrogen might explain the different effects of HRT on HDL-C level between the control and overweight groups. However, we did not measure the levels of female sex hormones. Further study will be needed to confirm our hypothesis.
In conclusion, HRT in overweight postmenopausal women is less effective in elevating HDL-C levels than in postmenopausal women with normal body weight. As patients with coronary artery disease are frequently overweight, the favorable effects of HRT on lipid profiles in overweight postmenopausal women may not be sufficient to overcome the adverse effects. This finding might partially explain no cardioprotective effects of HRT in randomized prevention studies.