The Relationship between Serum VEGF Concentration and Prognosis of Lung Cancer
Article information
Abstract
Background:
VEGF is an important factor for angiogenesis. Although many previous studies have reported an increased serum VEGF concentration in various malignant tumors, there are few studies on the relationship between serum VEGF concentration and its prognosis. This study investigated whether serum VEGF concentration is a prognostic indicator for lung cancer.
Methods:
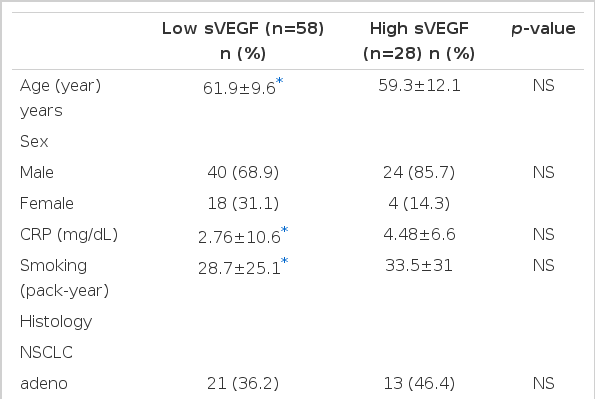
Using the ELISA kit, we measured the serum VEGF concentrations of 86 patients diagnosed with lung cancer on histologic examination. With a cut-off value of 686 pg/mL, the patients were classified as low-concentration (<686 pg/mL, n=58) or high-concentration (≥686 pg/mL, n=28) based on their mean serum VEGF concentration values to compare survival rates, and serum VEGF concentrations for different histologic types and stages.
Results:
There was no significant difference in serum VEGF concentration based on stage and histologic type between the two groups. Moreover, there was no significant difference in survival rate between the high-concentration and low-concentration groups (p=0.86).
Conclusion:
This study demonstrates that serum VEGF concentration is not associated with the prognosis of lung cancer.
INTRODUCTION
Angiogenesis plays an important role not only in non-neoplastic diseases such as diabetic retinopathy and arthritis1, 2), but also in the growth and metastasis of malignant neoplasms3). It has been known that such substances as transforming growth factor β (TGF-β), tumor necrosis factor α (TNF-α), platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) are involved in angiogenesis and are secreted by tumor cells, vascular endothelial cells and inflammatory cells4–6).
VEGF, in particular, increases the vascular permeability such that the plasma protein can easily leak out of the vessels7, 8), and the permeated plasma protein induces proliferation of tumor cells and vascular endothelial cells by forming fibrin gel9, 10). Many studies have reported that VEGF expression is associated with the malignancy of tumors. There have been reports in Korea on the association of VEGF expression with angiogenic status, malignancy and survival rate in breast cancer11), stomach cancer12) and lung cancer13). However, the results were not all consistent. Moreover, there was no effect on the patients who did not receive surgery, since most of the studies determined VEGF expression by using the immunochemical staining of the neoplastic tissue. A recent study has reported that serum VEGF (sVEGF) concentration is significantly correlated with intratumoral VEGF and intratumoral microvessel density, stating that sVEGF concentration could be used as a prognostic indicator for angiogenic status of tumors14). Accordingly, we have closely examined whether sVEGF concentration can be used as a biological prognostic factor for lung cancer by evaluating the correlation of sVEGF concentration with histologic type, stage, and survival rate in patients with lung cancer.
MATERIALS AND METHODS
1. Subjects
Eighty-six patients admitted to Kangbuk Samsung hospital in Seoul, Korea between January 1, 2000 to December 31, 2001 and diagnosed with lung cancer on bronchoscopy, percutaneous fine-needle aspiration or sputum cytology were selected for the study. We retrospectively reviewed their medical records to examine their clinical characteristics and survival periods. The mean age was 60.7 years, with 67 males and 19 females participating. Our histopathologic findings showed 65 cases of non-small cell lung cancer 21 cases of small cell lung cancer. Among the non-small cell lung cancer patients, 26 were squamous cell carcinoma, 34 were adenocarcinoma, and 5 were other cases (2 large cell carcinoma, 2 carcinoid tumor, and 1 bronchioloalveolar carcinoma). The stage distribution for non-small lung cancer patients were as follows: 1 Stage I, 1 Stage IIA, 3 Stage IIB, 8 Stage IIIA, 25 Stage IIIB, and 27 Stage IV. On the other hand, two stages were observed for the small cell lung cancer patients: limited stage (6 cases) and extensive stage (15 cases).
2. Methods
The patients blood was kept at room temperature for over 30 minutes and was centrifuged at 2000 g for 10 minutes, and then kept at −70°C. The sVEGF concentration was measured by using the ELISA kit (R&D Systems, USA). After diluting the serum to 1:20 and adding 100 μL of diluted conjugate in each well, we added 100 μL of standard, control sample and kept it at room temperature for 1.5 hours before absorbing and rinsing 6 times. We then added an extra 100 μL of substrate, followed by 100 μL of stop solution after 30 minutes at room temperature. By measuring the color development at 450 nm with a microtitre plate reader (Labsystems, Finland) within 30 minutes, we calculated the pg/mL sVEGF concentration. Patients were classified into two groups based on whether their mean sVEGF concentration value was above or below 686 pg/mL: the low-concentration group (<686 pg/mL, n=58) and high-concentration group (≥686 pg/mL, n=28).
3. Statistical Analysis
Statistical analysis was performed using the SPSS program. We used one-way ANOVA tests to compare the values of each group, and the Bonferroni test was employed for post hoc comparisons. Patients’ survival rates were tested using the Kaplan & Meier method;. they were determined to be significant at the value of p<0.05.
RESULTS
Analysis of the two groups showed that the mean sVEGF concentrations were 341 pg/mL in the low-concentration group and 1,400 pg/mL in the high-concentration group (p < 0.05) (Table 1). There were no significant differences between the two groups in terms of age, sex, histologic type and stages. The high-concentration group did have longer smoking records and higher levels of C-reactive protein with no significant (p=0.43, p=0.44).
The sVEGF concentrations showed no significant difference by stage, but they tended to increase for non-small cell lung cancer patients as the stages progressed (Figure 1). For small cell lung cancer patients, the sVEGF concentration was high in the limited stage compared to the extensive stage, which was not significant (671 pg/mL vs. 579 pg/mL, p=0.72). While mean sVEGF concentration was 782 pg/mL in adenocarcinoma, 680 pg/mL in squamous cell carcinoma, and 605 pg/mL in small cell lung cancer, with adenocarcinoma being the highest, there was no significant difference within each histologic type (Figure 2). Overall, the median survival period for the 86 patients was 13.8 months, with 55% surviving for more than one year. The median period of survival for the low-concentration group was 13.9 months and 13.3 months for the high-concentration group, showing nosignificant difference between the two groups (p=0.86) (Figure 3).

Mean concentrations of VEGF according to stage of non-small cell lung cancer (NSCLC, left) and small cell lung cancers (SCLC, right). All p-values between separate groups were not significant.
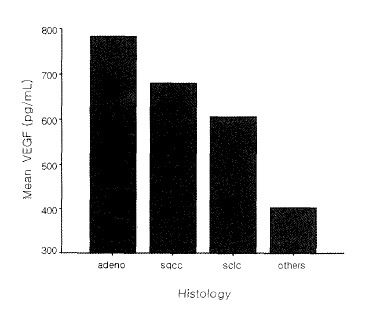
Mean concentration of VEGF according to histology. All p-values between separate groups were not significant, adeno, adenocarcinoma; sqcc, squamous cell carcinoma; sclc, small cell lung cancer; others, contain large cell carcinoma, carcinoid tumor and bronchioloalveolar carcinoma.
DISCUSSION
Many studies have reported that VEGF expression is evidently involved in the angiogenesis of tumor cells. VEGF expression has also been associated with malignancy as well as the survival rate of some malignant tumors15, 16). In addition, some animal experimental models have shown that angiogenesis and tumor cell growth increased with VEGF administration17, 18) while injecting anti-VEGF antibodies19). Inhibited tumor growth. In a study on patients with lung cancer, Tamura et al.14) reported that sVEGF concentration was higher in patients with lung cancer than in the normal controls and was correlated with intratumoral VEGF, stating that sVEGF concentration is an indicator for angiogenic status of tumor cells. In another study, sVEGF concentration decreased significantly after chemotherapy in a response group while no-response group with lung cancer exhibited no change20). However, in this study, sVEGF concentration displayed a tendency to increase with stage progression, while it registered no significant difference in each of the stages. At the same time in the survival rates between the low-concentration and high-concentration groups were relatively similar. The mean sVEGF concentration for of the patients was 686 pg/mL higher than other previous studies, as 81% of the patients were above stage IIIB.
The association of VEGF expression with angiogenic status and prognosis of lung cancer has been controversial. Some studies reported that VEGF correlated with angiogenesis but didnot associated with neoplastic proliferation in squamous cell carcinoma21), whereas increased VEGF expression was associated with high angiogenic status and poor prognosis in adenocarcinoma22). This study revealed that sVEGF concentration was high in adenocarcinoma in comparison with other histologic types, if not significant, which was similar to the study by lmoto et al.23) that reported VEGF expression to be higher in adenocarcinoma than in squamous cell cacinoma. In a study on patients with small cell lung cancer, Salven et al.24) reported that prognosis was poor for patients with high sVEGF concentration or at the extensive stage prior to anti-cancer chemotherapy positing that sVEGF as well as stage was attributable to prognostic indication. However, our study showed that sVEGF concentration was relatively high for the limited stage, indicating that there was no correlation between sVEGF concentration and stage in small cell lung cancer patients.
These discrepancies are assumed to be due to the following reasons. First, angiogenesis is caused by interaction between various activators and inhibitors for angiogenesis, other than VEGF. Second, VEGF acts as an autocrine or paracrine growth factor, rather than an endocrine growth factor in malignant tumors therefore, sVEGF concentration is not an indicator for tumor growth and angiogenesis. Third, sVEGF concentration may be affected by leukocytes and C-reactive protein in such diseases as infection and chronic inflammation. Fourth, since 81% of the patients were over stage IIIB in this study, the survival rate rather than sVEGF concentration was greatly affected by the patient’s nutritional status, presence of accompanied diseases and remote metastasis.
Based on previous reports that VEGF expression correlates with the prognosis of lung cancer, we examined the correlation of sVEGF with stage, histologic type, prognosis and survival rate. However, we found that there was no correlation between them. Hence, we conclude that sVEGF concentration is not associated with the prognosis of progressive lung cancers, but further studies are needed for complete verification.