What is the Clinical Significance of Transudative Malignant Pleural Effusion?
Article information
Abstract
Background:
A few reports of transudative malignant effusion on a small number of patients have suggested the need to perform routine cytologic examination in all cases of transudative pleural effusion, whether encountered for malignancy or not. The purpose of this study was to investigate whether cytologic examination should be performed in all cases of transudative pleural effusion for the diagnosis of malignancy.
Methods:
We performed a retrospective study of 229 consecutive patients with malignant pleural effusion, proven either cytologically or with biopsy. In patients with transudative pleural effusion, we reviewed medical records, results of transthoracic echocardiography, fiberoptic bronchoscopy, chest X-ray, chest CT scan, and ultrasonogram of the abdomen. These data were examined with particular attention to identifying whether or not the malignancy was suggested on chest X-ray, examining the involvement of the superior vena cava, great vessels, and lymph nodes, determining the presence of pericardial effusion, and observing the endobronchial obstruction.
Results:
Transudative malignant pleural effusion was observed in seven (3.1%) of the 229 patients, and was caused either by the malignancy itself (6 patients) or by coexisting cardiac diseases (1 patient). All the patients showed evidence suggesting the presence of malignancy at the time of initial thoracentesis, which facilitated the decision of most clinicians on whether to perform cytologic examination for the diagnosis of malignancy.
Conclusion:
Therefore, in all cases of transudative pleaural effusion, no clinical implications indicating malignancy were found on cytologic examination.
INTRODUCTION
After the initial differentiation between transudates and exudates in the diagnosis of pleural effusion, further diagnostic evaluation was traditionally thought to be needless in cases of transudative pleural effusion. Recently a few reports have stated that transudative effusion may be observed in malignant pleural effusion and have suggested the need to perform routine cytologic examination in all cases of transudative pleural effusion1–6). In this study, we attempted to evaluate the prevalence of transudates among malignant pleural effusion, proven either cytologically or with biopsy, to elucidate the etiology causing the transudative pleural effusion, and to determine whether the presence of transudative malignant pleural effusion has clinical implications for its diagnostic evaluation.
MATERIALS AND METHODS
From January 1999 to June 2001, 278 consecutive patients with malignant pleural effusion were admitted to the pulmonary department of the teaching hospital. After excluding 49 patients with incomplete data, the study population consisted of 229 patients with malignant pleural effusion, proven either cytologically or via biopsies. The present study was limited to the data from pleural effusion at the first thoracentesis. In the application of Light’s criteria for differentiating transudate from exudate, exudative effusion was defined as meeting at least one of the following three criteria: 1. pleural fluid LDH level ≥ 2/3 of the upper limits of the normal serum LDH value; 2. pleural fluid LDH/serum LDH value>0.6; and 3. pleural fluid protein/serum protein level>0.56). According to the decision of the attending physician, the liver, heart, kidney and thyroid were studied in transudative pleural effusion. We reviewed medical records, laboratory results, such as thyroid function test, levels of blood urea nitrogen, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and albumin, as well as the results from transthoracic echocardiography (TTE) and fiberoptic bronchoscopy, in patients with transudative pleural effusion. The results from radiologic studies were reviewed by chest X-ray, chest CT scan, and ultrasonogram of the abdomen on the day of thoracentesis. At the beginning of the diagnostic work up of transudative pleural effusion, we evaluated whether the malignancy was suggested on chest X-ray by the radiologist or clinician, or whether the malignancy had already been diagnosed. We also examined the involvement of the superior vena cava, great vessels, and lymph nodes, studied the presence of pericardial effusion seen on chest CT scan or TTE, and observed the occurrence of endobronchial obstruction or obstructive pneumonitis on fiberoptic bronchoscopy or chest CT scan.
RESULTS
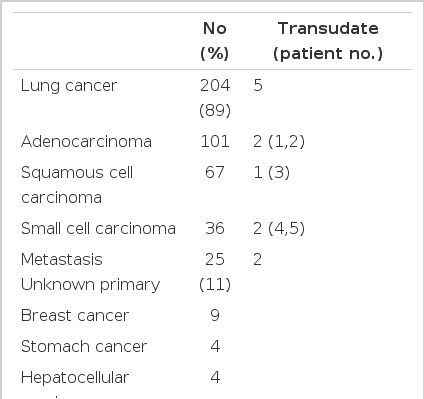
In our series, primary lung cancer was the most common cause of malignant pleural effusion, diagnosed cytologically or with biopsies, affecting 204 (89%) of the 229 patients. Unknown primary adenocarcinoma was also common from metastases (Table 1).
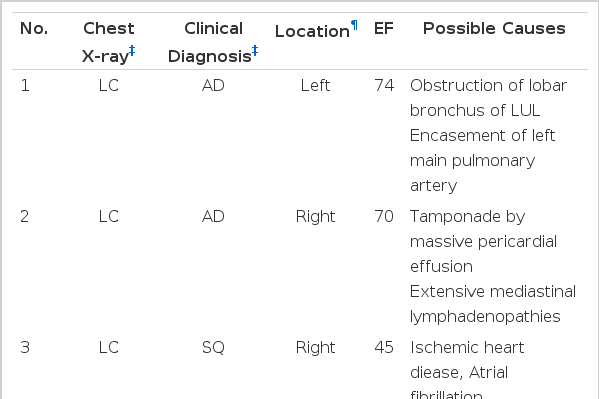
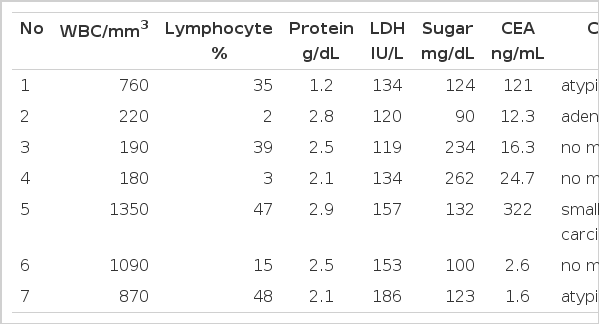
Transudative pleural effusion was observed in 7 (3.1%) of the 229 patients with malignant disease (Table 2). Five patients had primary lung cancer: adenocarcinoma for patients nos. 1–2, squamous cell carcinoma for no. 3, and small cell carcinoma for nos. 4 and 5. Their clinical stages were stage IIIB in all patients. Patient no. 6 had bladder carcinoma with lymphangitic metastasis to both lungs at the time of diagnosis, while patient no. 7 had invasive thymoma. Of the 7 patients, 4 (nos. 4–7) had pleural effusion on initial chest X-ray. In the other 3 patients, pleural effusion was not observed initially, but was disclosed on follow-up chest X-ray after the diagnosis of malignancy. No patients had abnormal values for blood urea nitrogen, creatinine, AST, or ALT on the day of thoracentesis, and none had a history of liver disease or thyroid disease. Lower levels of serum albumin were observed in one patient, no. 1 at 2.7 g/dL (normal range: 3.1–5.2 g/dL) on the day of thoracentesis. Thyroid function was evaluated in one patient (no. 1) with normal results. Abdominal ultrasonogram revealed normal findings in all patients. The level of pleural fluid carcinoembryonic antigen (CEA) was more than 10 ng/mL in 5 patients (nos. 1–5) (Table 3). At initial thoracentesis, the presence of malignancy was suggested for all patients, by chest X-ray or other clinical information.
DISCUSSIONS
As in other countries, lung cancer is the most common cause of cancer death in Korea. Malignant pleural effusion is defined as pleural effusion associated with cancer and has been a common clinical problem encountered by most clinicians. However, the prevalence of transudates has been rarely reported in malignant pleural effusion. After the initial study by Light et al.6) stating the prevalence of malignant transudative pleural effusion as 2.3% for 43 patients, the prevalence has been reported to range from 1 to 10.6% in other studies1–5). In the largest study to date, by Ashchi et al7), the prevalence was reported as 4.6% among 171 malignant pleural effusion patients. Our series showed a prevalence of transudative malignant pleural effusion of 3.1% for the 229 patients.
The following causes of transudates in malignant pleural effusion have been recognized: 1) malignancy itself 2) coexisting diseases and 3) combination of both causes. Transudative pleural effusion may occur in the early stage of malignant pleural effusion and show a lower protein level of pleural fluid because of the involvement of the mediastinal lymph node. The pleural fluid characteristics changed to exudates with protein accumulation in the pleural space over a period of several weeks. Endobronchial obstruction or hypoalbuminemia also causes transudative pleural effusion in patients with malignant disease8–11). This unusual condition was also observed in patients with superior vena cava syndrome or tumor embolism12). Coexisting diseases such as cardiac, renal, and hepatic diseases have been well known to cause transudative pleural effusion. In our series, of 7 patients with transudative pleural effusion, 6 may have been caused by pericardial effusion, mediastinal involvement of the malignancy, and obstruction of the bronchus and great vessels in other words, by the malignancy itself. Cardiac disease as a coexisting condition was thought to be the major cause in one patient.
The clinical decision of whether cytologic examination should be performed in all patients of transudative pleural effusion for the diagnosis of malignancy has remained controversial. While some investigators2, 3, 5) suggest the need for cytologic evaluation in transudative effusion, others4, 13) do not. Where malignancy has already been diagnosed, the need for cytologic evaluation in patients of transudative pleural effusion may be absent, with the exception of the purpose of staging lung cancer. Furthermore, with initial chest X-ray suggesting the presence of malignancy, the decision by most clinicians to perform cytologic examination for diagnosis is straightforward. Therefore, previous studies were limited by the absence of any discussion regarding whether the malignancy had been suggested on chest X-ray at the time of thoracentesis. Moreover, to our knowledge, this study was conducted with the largest number of patients with malignant pleural effusion to identify the value of cytologic examination in transudative pleural effusion. For the 4 patients with transudative pleural effusion on admission in our series, the clinicians were already aware of the possible presence of malignancy from the chest X-rays and through other clinical information. In addition, the other 2 patients had already been diagnosed with primary lung cancer at the time of thoracentesis. From our series, we conclude that there was no clinical implication indicating the need for cytologic examination to diagnose malignancy in all patients of transudative pleural effusion encountered.
Acknowledgements
This work was supported by an Inha University Research Grant (INHA-30172).