The Inverse Association Between the Presence of Antibody to Hepatitis B Surface Antigen and Atopy in Young Adults
Article information
Abstract
Background
Some bacterial and viral infections may reduce the risk of atopy, and this is based on the concept of their ability to divert the immune system towards the Th1 responses. Most of the hepatitis B virus (HBV) infections occur in the developing countries and this is where atopic disorders are least prevalent. Th1 responses are important for the viral clearance of HBV and also for antibody production. The aim of the study is to investigate whether the presence of antibodies to the hepatitis B surface antigen (anti-HBs) is inversely associated with atopy in adults.
Methods
A random sample of 358 subjects, who were without hepatitis B surface antigen, was recruited; they were aged from 18 to 79 years (105 young adults aged ≤40 years and 253 older adults aged > 40 years). Determinations of the anti-HBs and skin prick tests using aeroallergens were performed. Those subjects with one or more positive skin reactions (a mean wheal diameter ≥3 mm) were considered atopic.
Results
The prevalence rate of atopy (p=0.035) or the sensitization to Dermatophagoides farinae (p=0.01) was significantly lower in the subjects with anti-HBs than in those subjects without anti-HBs for the young adults, but not for the older adults. The logistic regression analysis that was done on the young adults showed that the presence of anti-HBs was associated with a significantly lower risk of atopy (the odds ratio adjusted for confounding variables = 0.40 [95% CI 0.16-0.98], p=0.046) or with the sensitization to D. farinae (0.20 [0.06-0.65], p = 0.008).
Conclusion
The presence of anti-HBs produced by a natural HBV infection or vaccination might be inversely associated with atopy in young adults.
INTRODUCTION
Atopy is the the ability to produce IgE to the common aeroallergens such as those derived from house dust mites and pollens, and it is considered to be one of the key factors in developing asthma, hay fever and eczema1). The prevalence of atopic disorders is generally low in developing countries, but the prevalence has increased in the industrialized world in the past few decades2). The reasons for this increase are largely unknown, but the concurrent improvement of sanitation and the reduction in childhood infections in developed countries had led to the speculation that infections in early childhood may reduce the risk of allergy, and this is the so-called hygiene "hypothesis"3). Indeed, several epidemiological studies have shown an inverse association between bacterial (Mycobacterium tuberculosis) or viral (measles, hepatitis A) infections and atopy4-6). The hypothesis is based on the widely accepted concept of the divergence of differentiated effector T helper (Th) cells into two major subsets, the Th1 and Th2 cells that are largely mutually inhibitory and reciprocally regulated7). The Th1 cells, when stimulated with virus or intracellular bacteria, secrete interferon (IFN)-γ that can inhibit IgE production by the B cells7, 8).
More than 2 billion people, about one-third of the world's population, have been infected with hepatitis B virus (HBV). More than three-quarters of HBV infections occur in Asia, the Middle East, and Africa where the atopic disorders are the least prevalent2). Thus, certain features of HBV infections might well contribute to the lower rate of allergies in the developing countries. A great deal of information on the role of cytokines during HBV infection has been accumulated9). Indeed, several studies have demonstrated that the Th1 cytokines, IL-12 and IFN-γ, are important factor for viral clearance in HBV infection10-12). The HBV vaccine, which has been introduced into national immunization programms in some high endemic countries13), is reported to activate the antigen-presenting dendritic cells and to increase the IL-12 production14). Hepatitis B surface antigen (HBsAg) represents the envelope protein of HBV, and the antibodies to HBsAg (anti-HBs) develop several weeks to months after viral clearance15). It could be hypothesized that individuals with anti-HBs antibodies after a natural HBV infection or vaccination have a more polarized Th1 immune response, and so they are less likely to have atopy.
We investigated whether the presence of anti-HBs antibodies was inversely associated with atopy in a cross-sectional study of an adult population.
MATERIALS AND METHODS
Population sample
A random sample of 502 adults was asked to participate in the present cross-sectional study. They visited the Health Screening Center at the Chonnam National University Hospital for a medical checkup and they live in Gwangju City and in Chonnam Province, which are located in the southwestern area of South Korea. The three-hundred and eighty-four subjects who agreed to take a skin prick test with common aeroallergens were included in this study. Because we set out to analyze the prevalence of atopy according to the presence or the absence of anti-HBs, the 26 subjects that had HBsAg were excluded from the study. The final number of subjects selected for this study was 358, and this corresponded to 71.3% of those people visiting the Health Screening Center. There were 240 male and 118 female subjects, and their ages ranged from 18 to 79 years (mean±SD: 48.1±11.8). There were no differences for age, gender, body mass index and smoking habits between the subjects who were included and those who were not included in this study. The present study was approved by the Human Ethics Review Committee of our University Hospital, and an informed consent was obtained from all subjects.
Clinical characteristics
Demographic data and information on atopic disorders within the first degree of relatives and a prior history of antituberculosis chemotherapy were obtained. The other evidences of past exposure to mycobacteria included an old Bacille Calmette-Gurin scar in deltoid area and old healed tuberculosis lesions on chest radiographs. The radiographs were interpreted by an independent radiologist who was unaware of the study, and the somewhat increased fibrostreaky densities on X-ray were considered as healed tuberculosis lesions. The subjects' height and weight were measured and the body mass index was calculated as kg/m2.
Skin prick tests
Skin prick tests were performed as previously reported16) on the volar side of each individual's lower arm with the following common aeroallergens: Dermatophagoides farinae, mixed moulds (Aspergillus fumigatus, Mucor mucedo, Penicillium notatum, Pullularia pullulans, Rhizopus nigricans, Serpula lacrymans), dog, mixed trees (beech, birch, oak, plane tree), and mugwort (Allergopharma, Reinbek, Germany). A wheal reaction greater than or equal to 3 mm after subtraction of the negative control reaction was regarded as positive. Subjects with one or more positive reactions were considered atopic.
Tests for infectious diseases
Medical testing for some of the infectious diseases that was done in the center included serologic tests for HBsAg, anti-HBs, antibody to the hepatitis C virus and antibody to the human immunodeficiency virus, and a stool examination for parasite eggs. HBsAg, anti-HBs, antibody to hepatitis C virus and antibody to human immunodeficiency virus were determined by commercial microparticle enzyme immunoassays (ABBOTT Laboratories, Abbott park, IL). Positivity or negativity was assigned according to the kit's instructions. Stool samples were analyzed qualitatively for parasite eggs by the cellophane thick smear method.
Statistical analysis
We analyzed data using the Statistical Package for the Social Sciences (SPSS) for Windows Release 12.0.0 (SPSS inc. Chicago, IL). The results were expressed as the number of subjects (percentages) for the dichotomous variables and as means±SD for the continuous variables. Comparisons were made using χ2 tests for the dichotomous variables and using Student's t-test for the continuous variables. To estimate the independent effect of the presence of anti-HBs on atopy, the adjusted odds ratios were calculated by logistic regression analysis with the enter method. p values < 0.05 were considered statistically significant.
RESULTS
This study population included 105 young adults aged less than or equal to 40 years old and 253 older adults aged greater than 40 years old. Two-hundred forty-five (68.4%) subjects had anti-HBs and 113 subjects (31.6%) had no antibody. One-hundred sixty-two subjects (45.3%) had the history of HBV vaccination; 124 (76.5%) subjects had the antibody and 38 subjects (23.5%) had no antibody. Antibody to hepatitis C virus and parasite eggs were detected in 4 (1.1%) and 15 (4.2%) subjects, respectively. The parasites included Ascaris lumbricoides, Clonorchis sinensis, and Metagonimus yokogawai, and these parasites were detected in 1 (0.3%), 11 (3.1%), and 3 (0.8%) subjects, respectively. The testing for antibody to human immunodeficiency virus was negative in all the subjects.
There were no differences in age, gender, body mass index, area of residence, smoking habits, atopic disorders within the first degree of relatives, antibody to hepatitis C virus, parasite eggs in stool, and evidences of prior exposure to mycobacteria between the positive and negative anti-HBs groups (Table 1).
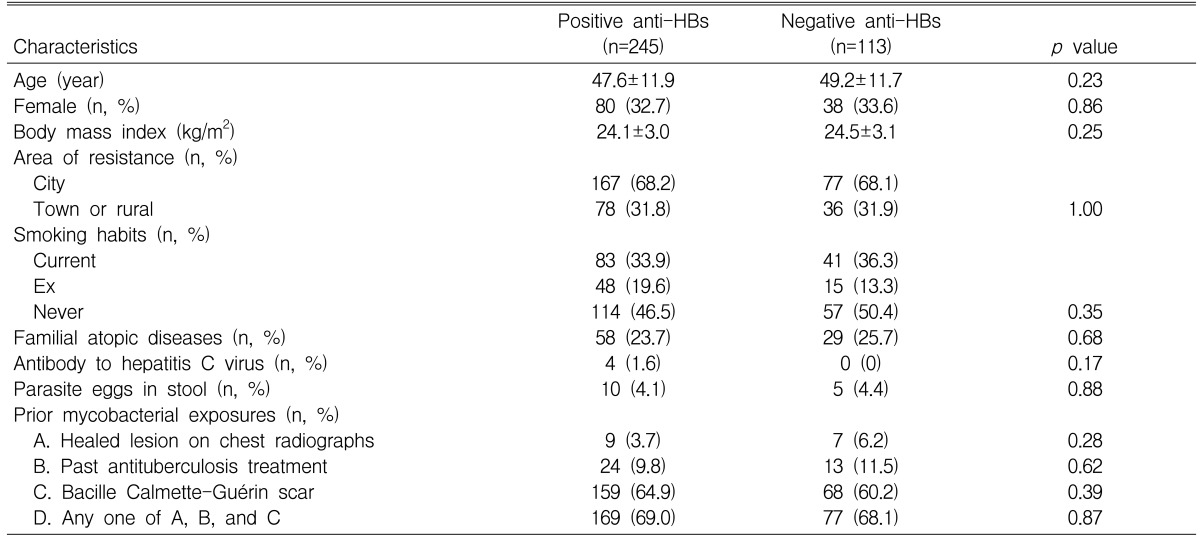
Characteristics of subjects with and without antibodies to hepatitis B surface antigen (anti-HBs) in all subjects
One-hundred and eighteen (33.0%) subjects were atopic on the skin prick tests. There were no significant differences for gender, body mass index, area of residence, smoking habits, atopic disorders within the first degree of relatives, antibody to hepatitis C virus, parasite eggs in stool and evidences of prior exposure to mycobacteria between the atopic and nonatopic subjects (p>0.05, respectively), although the mean age was lower in the atopic subjects than in the nonatopic subjects (45.5±11.0 years versus 49.4±12.0 years, p<0.05). The prevalence rate of atopy did not differ between the positive and negative anti-HBs groups (77 [31.4%] versus 41 [36.3%], p=0.36). When the skin reaction to each allergen was analyzed, the prevalence rate of sensitization to D. farinae was significantly lower in the positive anti-HBs group than in the negative anti-HBs group (27 [11.0%] versus 22 [19.5%], p=0.03). There were no differences in the prevalence rates of the sensitization to dog (24 [9.8%] versus 11 [9.7%], p=0.99), the mould mixture (15 [6.1%] versus 10 [8.8%], p=0.35), the tree mixture (28 [11.4%] versus 20 [17.7%], p=0.11), and mugwort (28 [11.4%] versus 17 [15.0%], p=0.34) between the two groups.
When the analyses were limited to young adults, 73 (69.5%) of 105 subjects had anti-HBs and 32 (30.5%) of 105 subjects had no antibody. There were no differences in the known variables between the positive and negative anti-HBs groups (Table 2). Forty-three (41.0%) subjects were atopic on the skin prick tests. There were no significant differences in the known variables between the atopic and nonatopic subjects (p>0.05, respectively). The prevalence rate of atopy was significantly lower in the positive anti-HBs group than in the negative anti-HBs group (p=0.035). The prevalence rate for the sensitization to D. farinae was significantly lower in the positive anti-HBs group than in the negative anti-HBs group (p=0.01). The prevalence rates for the sensitization to dog, the mould mixture, the tree mixture and mugwort were lower in the positive anti-HBs group than in the negative anti-HBs group, although this was not significant (p>0.05, respectively) (Figure 1A). Logistic regression analysis showed that the presence of anti-HBs was associated with a significantly lower risk of atopy (odds ratio 0.40 [95% CI 0.16-0.98], p=0.046) or the sensitization of D. farinae (0.20 [0.06-0.65], p=0.008), after adjustment was made for variables such as age, gender, area of residence, body mass index, current smoking, familial atopic disorders, parasite infestation and evidences of past exposure of mycobacteria.
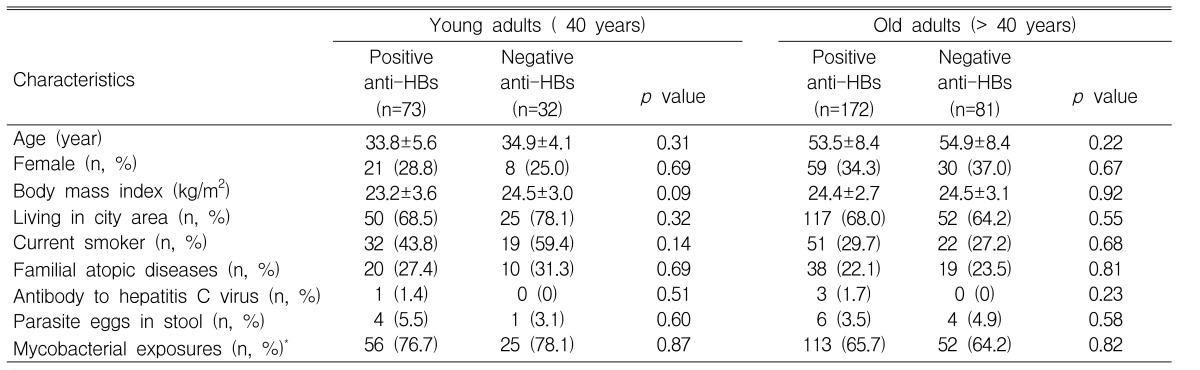
Characteristics of subjects with and without antibodies to hepatitis B surface antigen (anti-HBs) in young or old adults
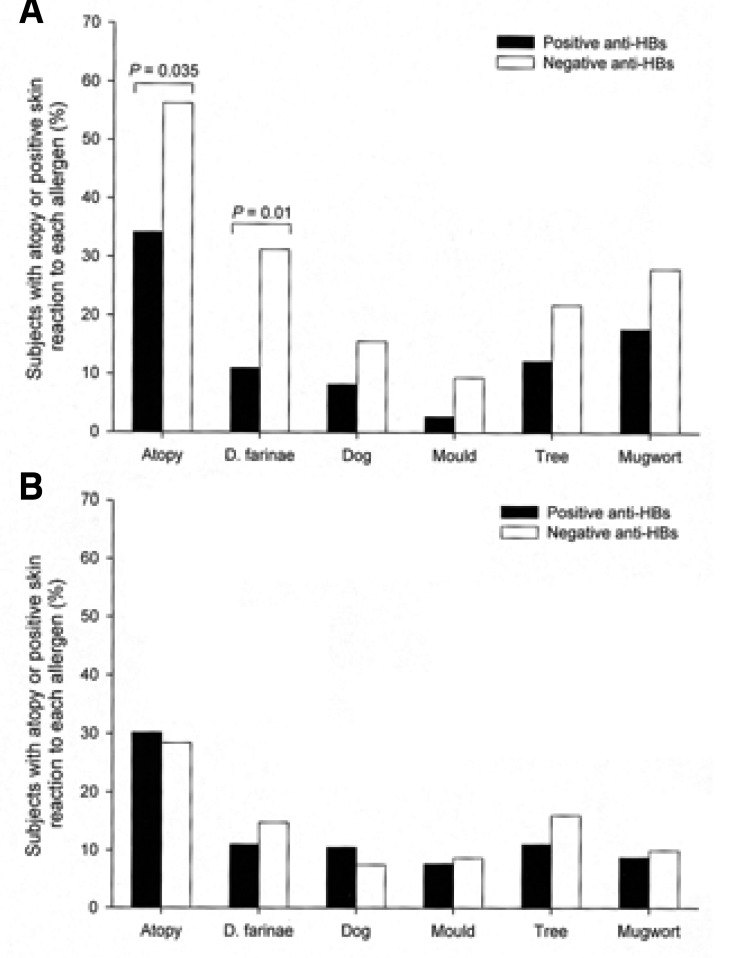
Comparison of the prevalence rate of atopy or the sensitization to each allergen between the positive and negative anti-HBs groups for the young (A) or older (B) adults.
When the analyses were limited to older adults, 172 (68.0%) subjects had anti-HBs and 81 subjects (32.0%) had no antibody. There were no differences in the known variables between the positive and negative anti-HBs groups (Table 2). Seventy-five (29.6%) subjects were atopic on the skin prick tests. There were no significant differences in other known variables between the atopic and nonatopic subjects (p>0.05, respectively), although the mean age was lower for the atopic subjects than for the nonatopic subjects (52.3±7.0 years versus 54.7±8.9 years, p<0.05). There were no differences in the prevalence rates of atopy and the sensitizations to D. farinae, dog, the mould mixture, the tree mixture and mugwort between the positive and negative anti-HBs groups (p>0.05, respectively) (Figure 1B).
When the analyses were limited to all those subjects with the history of HBV vaccination, the prevalence rate for atopy did not differ between the 124 subjects with anti-HBs and the 38 subjects without anti-HBs (41 [33.1%] versus 15 [39.5%], p>0.05). The prevalence rate for sensitization to D. farinae was significantly lower in the positive anti-HBs group than in the negative anti-HBs group (13 [10.5%] versus 10 [26.3%], p=0.01). There were no differences in the prevalence rates for the sensitization to dog, the mould mixture, the tree mixture and mugwort between the two groups (p>0.05, respectively). When the analyses were limited to the young subjects with the history of HBV vaccination, the prevalence rate of atopy did not differ between the 40 subjects with anti-HBs and 12 subjects without anti-HBs (13 [32.5%] versus 6 [50.0%], p>0.05). The prevalence rate of sensitization to D. farinae was significantly lower for the positive anti-HBs group than for the negative anti-HBs group (2 [5.0%] versus 4 [33.3%], p=0.01). There were no differences in the prevalence rates for the sensitization to dog, the mould mixture, the tree mixture and mugwort between the two groups (p>0.05, respectively).
DISCUSSION
In the present study, we showed the negative association between the presence of anti-HBs and atopy or the sensitivity to D. farinae, which is known to be the most common allergen in this country in young adults, although this inverse association was not maintained in the older adults. To the best of our knowledge, this is the first study to show that the presence of anti-HBs produced by a natural HBV infection or vaccination may be inversely associated with atopy. This finding might partly explain why atopic disorders are least prevalent in Asia and Africa2), where the prevalence of HBV infection has been higher, and in some countries where the HBV vaccine has been introduced into their national immunization programmes13).
It is known that the immune response to HBV is responsible both for viral clearance and for the pathogenesis of the disease during HBV infection9). During the natural course of chronic HBV infection, some patients undergo a spontaneous exacerbation of the liver damage with an elevation of serum aminotransferases, and this may result in seroconversion of the hepatitis B e antigen (HBeAg) to the antibody to HBeAg (anti-HBe), and it also results in viral clearance. These hepatitis flare-ups are associated with the enhancement of the virus-specific T helper cell reactivity17-19). Rossol et al.12) have recently shown that a substantial increase in IL-12 production, along with the induction of Th1 cytokines such as IFN-γ and IL-2, is required for the sustained immune control over HBV replication, and this is manifested by seroconversion to anti-HBe. In the transgenic mouse models, it has been demonstrated that IL-12 can suppress HBV-replication by the induction of IFN-γ10, 11). The HBeAg begin to fall at the onset of illness and it may be undetectable at the time of the peak clinical illness. HBsAg becomes undetectable and anti-HBs arise during recovery, several weeks to months after the loss of HBeAg. Anti-HBs is a long-lasting antibody and it is associated with immunity15). Accordingly, it might be considered that individuals with anti-HBs have a more enhanced Th1 type immune response compared with those individuals without the antibody, and they are less likely to have atopy. In contrast, a deficiency of the Th1 response to HBV and decreased levels of IL-12 and IFN-γ have been demonstrated in both HBV carriers and the chronic liver disease patients with HBV20, 21). Thus, the tendency to atopy might be increased for HBV carriers or for patients with chronic hepatitis B22). Due to this reason, the individuals with HBsAg were not included in the present study to keep any potential confounding to a minimum. Matricardi et al.23) have recently found that there was no significant association between the presence of antibodies to the hepatitis B core antigen and atopy. The fact that the antibodies to the hepatitis B core antigen may be detected not only in subjects who have recovered from hepatitis B, but also in those subjects with acute or chronic HBV infection might partly explain the negative finding.
Vaccination with HBsAg leads to the development of anti-HBs and this protects most normal individuals against HBV. The successful HBV vaccination is likely to induce the Th1 type immune responses14) and so might reduce the atopy. In South Korea, the national HBV vaccination program has been going since 198324). Our subjects with a history of HBV vaccination were probably vaccinated during their school period and after that, although it is inaccurate, 23.5% of them had an absent or poor antibody response. The failure to develop adequate antibodies is related to freezing the vaccine or to giving it into the buttock rather than the deltoid region25). A poor antibody response is also seen in the aged and in the immuno-compromised, including those people who are human immunodeficiency virus-positive25). All the subjects in the present study were human immunodeficiency virus-negative. In the present study, there was no significant association between the presence of anti-HBs and atopy in those young adults with the history of HBV vaccination, and this could be explained by the small sample size of studied subjects. However, there is a possibility that vaccination raises the risk for subsequent development of atopy by reducing the exposure to invasive childhood infections that are thought to strengthen the Th1 response26). A further prospective study is needed to shed more light on this.
It is interesting that the inverse association between the presence of anti-HBs and atopy was found in young adults, but not in the older adults. Although we did not obtain the information about the exact time when the subjects had natural HBV infections or vaccinations in our cross-sectional study, it could be speculated that a recent HBV infection-induced Th1 immune response reduces the Th2 response in young adults, but not in older adults. The allergen-specific Th2 memory cells may produce Th1 cytokines when they are activated in the presence of IL-12, a potent IFN-γ-inducing protein27). The reversibility of cytokine production in the memory CD4+ T cells, however, is lost after repeated stimulation, as the more malleable resting memory T cells differentiate into the end-stage effector cells28) in which the Th2 effector cells lose the IL-12 receptor function29). This reversibility might decrease or even disappear in older adults. Conventional allergen immunotherapy may increase the development of allergen-specific Th1 cells and also decrease the development of Th2 cells30). A study concerning house dust mite immunotherapy has demonstrated that the clinical improvement is inversely related to age. The subjects younger than 20 years of age were three times as apt to benefit from immunotherapy as those subjects who were older than 51 years31). The another possible explanation could be that a remote past HBV infection or a vaccination-induced Th1 immune response is maintained in young adults, but this decreases or disappears in older adults. The titer of anti-HBs is generally noted to decline to non-protective levels on prolonged follow-up. It has been demonstrated that anti-HBs titers are decreased to non-protective levels in at least 25~50% of vaccine recipients over a period of 5~10 years32, 33). In the case of either recent or the remote HBV infection or vaccination, the effects of the HBV-induced Th1 response on atopy might be weaken or lost all together in older adults. Additionally, it might be speculated that a long-standing exposure to other miscellaneous infections in older adults makes the effects of the HBV-induced Th1 response insignificant.
However, when considering that the strong Th1 response leads to the clearance of HBV and antibody production10-12), and the HBV carriers and the patients with chronic liver diseases may reflect the Th2-skewed response20, 21), an alternative explanation for our finding might be that the main association simply reflects the type of immune response that is present prior to HBV infection or vaccination, rather than any effect of the HBV exposures. If an individual is already Th1-skewed, they are more likely to generate anti-HBs and they are also likely to be less atopic.
We examined for potential confounding variables related to atopy. There were no differences in prior mycobacterial exposure6), parasitic infestation34), living area, family history and obesity35) between the subjects with and without anti-HBs. However, the information on the other potential confounders such as the family size, birth order36) and the socioeconomic level was not collected in the present study. These factors are likely to have affected our findings.
In the present study, the presence of anti-HBs was not significantly associated with the sensitivity to dog, mould, tree, or mugwort, except D. farinae. However, as shown in Figure 1A, the rates of sensitization to the allergens tended to be lower in the positive anti-HBs group compared with the negative anti-HBs group. The significant association might require a larger study.
When considering all the data together, the presence of anti-HBs produced by a natural HBV infection or by vaccination might be inversely associated with atopy in young adults. However, we need further prospective epidemiological and experimental studies, especially in children, to elucidate the possible interaction of HBV exposures with processes that lead to atopy.
ACKNOWLEDGEMENT
We thank the nursing and medical staff at the Health Screening Center at the Chonnam National University Hospital for their assistance, and we thank Jai-Dong Moon for the statistical advice.