Non-Hodgkin's Lymphoma Manifest as Gingival Hyperplasia in a Renal Transplant Recipient
Article information
Abstract
Gingival hyperplasia is a frequent complication in transplant patients who receive cyclosporine or calcium channel blockers. We studied an unusual case involving a renal transplant recipient with post-transplant non-Hodgkin's lymphoma that manifested as gingival hyperplasia. We initially consider that it was a side effect of cyclosporine and nifedipine. The lesion did not respond to dose reductions or the withdrawal of cyclosporine and nifedipine, and the gingival hyperplasia progressed in a localized fashion, becoming ulcerated and bleeding easily. Histological examination revealed the presence of malignant lymphoma.
INTRODUCTION
Gingival hyperplasia is common in renal transplant recipients treated with cyclosporine1-3). Gingival changes are seen in about 25% of patients medicated with cyclosporine, but the rate may increase to greater than 60% in patients medicated with both cyclosporine and a calcium channel blocker4-6).
In renal allograft recipients, the incidence of post- transplantation lymphoproliferative disorder (PTLD) is 2.3%7), and its overall annual incidence is 0.5%8). Up to 40% of PTLDs occur in extranodal sites such as the stomach, intestine, bone, central nervous system, eyes, and skin9, 10). Gingivae are rarely involved10).
The following case report relates a unique case of PTLD in a renal transplant recipient who was receiving cyclosporine and a calcium channel blocker, which manifested as gingival hyperplasia.
CASE REPORT
A male, 41 years old, visited the clinic in April 2002 after experiencing bleeding, pus discharge, edema, and pain of the gingivae over the previous three months (Figure 1A, 1B). He had previously been diagnosed with end stage renal failure because of IgA nephropathy. Additionally, continuous ambulatory peritoneal dialysis was initiated in January 1994. He underwent a kidney transplantation with 40 minutes of cold ischemia in November 1995. His brother, who had haploid-identical HLA typing, was the donor. The patient was maintained on medication consisting of 75 mg of cyclosporine twice daily, 12 mg of deflazacort once daily, and 750 mg of mycophenolate mofetile (MMF) administered twice daily. His post-operative progress was good until he developed gingival bleeding, discharged pus, and pain.

Gross findings in a patient with gingival hyperplasia. Compared with cyclosporine-induced gingival hyperplasia, the gingival lesion in this patient was characterized by a focal, irregular surface and ulceration.
Three months before entering our clinic, he was admitted to the outpatient dental clinic for treatment of hyperplastic gingivae (which at that time was considered to have been induced by cyclosporine). The lesion, however, did not improve despite scaling, oral antibiotics, and a reduction in the cyclosporine doses. Moreover, the gingival hyperplasia exhibited focal papillary growth and bled in response to light pressure. He had also lost 5 kg of body mass over the preceding six months.
A laboratory examination, including complete blood counts, blood chemistry, and urinary analysis, revealed that his hemoglobin concentration was 11.1 g/dL, blood urea nitrogen concentration was 27.5 mg/dL, and creatinine concentration was 1.46 mg/dL. The white cell count was 6370/µL while the platelet count was 256,000/µL. Other variables remained normal. Serum cyclosporine levels were low (Figure 2).
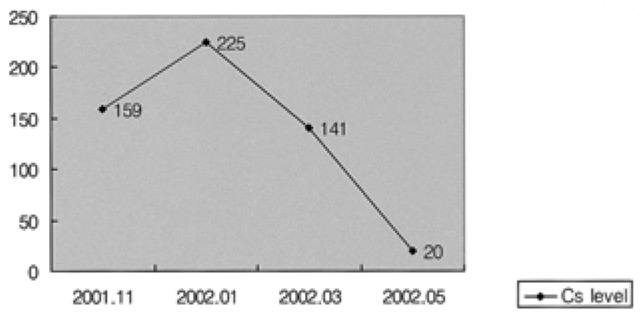
Serum cyclosporine levels in this patient decreased simultaneously with a reduction in the dose of cyclosporine from January 2002 onwards, but gingival hyperplasia was not alleviated despite this.
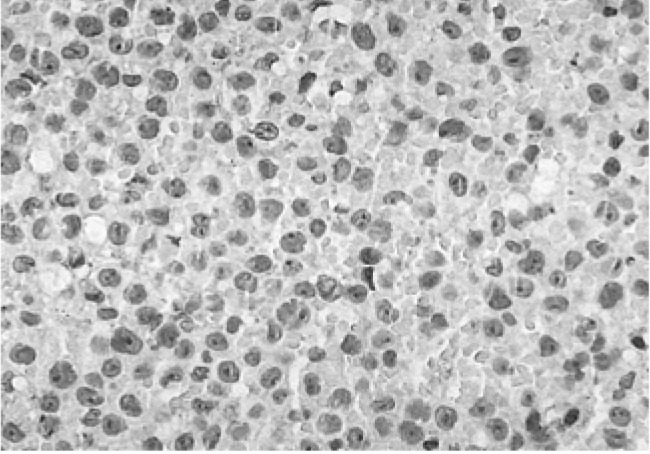
We did a biopsy of the lesion to identify other potential causes. The biopsy established the presence of leukemic cell infiltration including a malignant lymphoma or histiocytic tumor (Figure 3). Muramidase and immunohistochemical stains specific for LCA (Figure 4A), L26 (Figure 4B), UCHL1, and CD68 revealed a large diffuse B cell type of malignant lymphoma. The latent membrane protein for the Epstein-Barr virus (EBV-LMP) immunohistochemical stain was positive (Figure 5).
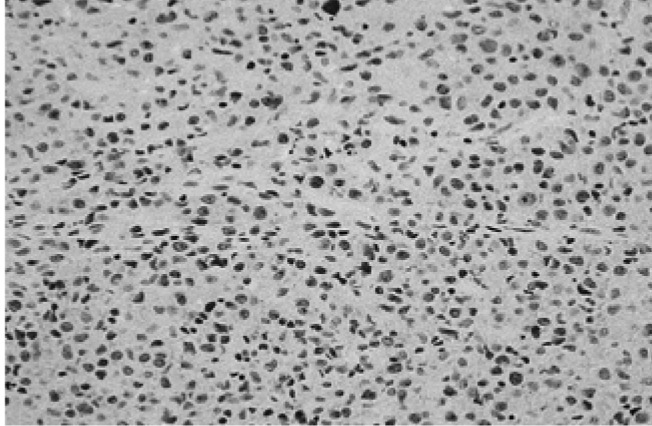
Microscopic image of a gingival biopsy showing atypical lymphocytes. These findings are indicative of the presence of leukemic cell infiltration and a malignant lymphoma or histiocytic tumor (×400, H-E stain).
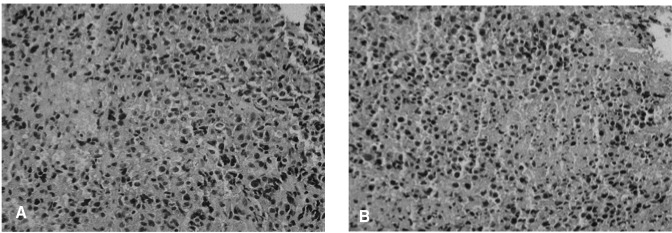
An immunohistochemical image of a tissue section showing positive reactions to LCA (A) and L26 (B) and negative reactions to UCHL-1 and CD68. This is indicative of a diffuse large B cell type malignant lymphoma (×400).
In the initial stage of the lymphoma, computed tomography (CT) scan of the neck indicated a 2×1.5 cm enlarged lymph node present in the right submandibular area several enlarged lymph nodes were also in the bilateral parapharyngeal space. Lung and intestinal CTs showed no evidence of lymphadenopathy. A bone marrow aspiration and biopsy revealed a slight hypocellularity (30%) and normal maturation. The final stage of the lymphoma was stage II (B) according to the Ann Arbor Staging System.
Administration of MMF and cyclosporine was stopped, but prednisolone treatment was continued. The patient started chemotherapy with a CHOP regimen of 1087 mg of cyclophosphamide (750 mg/m2), 72 mg of doxorubicin (50 mg/m2), 2 mg of vincristine, and 100 mg of prednisolone. After three serial CHOP chemotherapies, a neck CT showed a marked regression of the right submandibular lesion but no clearly enlarged lymphadenopathy in the other neck areas. CHOP therapy and external radiotherapy of the gingivae was performed a total of six times.
In 2004, the patient's gingivae were normal upon examination, and CT scans could not detect lymphadenopathy. Presently, his renal function is good and is treated by daily doses of 100 mg azathioprine and 7.5 mg prednisolone.
DISCUSSION
The clinical definition of gingival non-Hodgkin's lymphoma (NHL) varies, but it is usually described as an asymptomatic gingival enlargement or a mass of tissue that resembles a pyogenic granuloma10). Therefore, any diagnosis of PTLD could be missed or delayed if the physicians do not consider the possibility of lymphoma when assessing gingival overgrowth in patients receiving immunosuppressive drugs. In our case, we initially suspected cyclosporine or nifedipine as causing the gingival hyperplasia. However, this lesion was aggravated in spite of withdrawing from the nifedipine, reducing the dosage of cyclosporine (from 275 mg to 150 mg daily), and three months of conservative dental care. These findings suggested that other causes might have been responsible, and gingival biopsy eventually was revealed in NHL.
In retrospect, several of the initial symptoms were more indicative of lymphoma than drug-induced gingival hyperplasia. First, the patient had lost 5 kg of body mass during the preceding three months. Second, a neck CT revealed lymphadenopathy. Third, his gingival hyperplasia had progressed to a mass-like lesion that bled easily when touched. Had the possibility of a hidden malignancy been considered, a careful physical examination may have detected the lymphoma. Until now, only a few cases of gingival lymphoma or plasmacytoma in transplant patients10-14) have been reported. There was one case in a bone marrow transplant patient in 2000 and two cases after cardiac transplantations in 2004.
With progress in transplant medicine, the frequency of PTLDs is rising. The major risk factors for PTLD are the duration of immune suppression and the amount and number of immunosuppressive agents used4, 11, 15). These agents induce abnormal lymphoid cell proliferation, which may vary from the polymorphic proliferation of B lymphocytes to monoclonal high-grade malignant lymphoma16). Our patient had received dual therapy (cyclosporine and steroid) for seven years, followed by a regimen of MMF added 14 months ago to prevent chronic allograft nephropathy. There is currently no evidence to indicate that MMF is directly associated with the observed increase in PTLDs17-19), but the elevated intensity of immunosuppression by MMF may be partly responsible for PTLDs in this case.
The roles of EBV and its complications in the development of lymphomas have been studied extensively20-23). PTLDs may be the result of primary or reactivated EBV infections in a host with impaired immunity22). The EBV-positive rate in NHL is about 10%24). The major immunophenotype of PTLD is the B-cell lineage8). EBV infection is associated with 70% of B-cell PTLD cases25). In this case, we also confirmed the presence of EBV with in situ hybridization, since previous cases were usually associated with EBV.
Treatment of NHL in renal transplant recipients consists of reducing or withdrawing immunosuppressants and cytotoxic therapy (including chemotherapy and radiotherapy). Lymphoma of the gingivae was present in other patients with NHL. It has been reported that PTLDs subsequent to bone marrow transplantation were successfully treated by administering rituximab for four weeks and reducing the immunosuppressive agent doses10). Other patients with PTLD subsequent to cardiac transplantation were successfully treated by reducing the dose of the immunosuppressive agent11). Our patient was treated with conventional CHOP chemotherapy and radiotherapy because of the presence of several parapharyngeal lymphadenopathies and because a lack of response to a reduction in cyclosporine.
The prognosis of primary diffuse oral lymphomas is less favorable than that of nodular lymphomas26, 27), but both disease staging and early diagnosis are important prognostic factors. A good prognosis for PTLD is associated with early detection, localization to a single organ and treatment with cyclosporine, whereas late-onset PTLD, dissemination of the disease, and CNS or bone marrow involvement are associated with a poor prognosis28). Good and poor prognostic factors were present in our patient who had been treated with cyclosporine, but had developed late-onset PTLD.
In conclusion, non-Hodgkin's lymphoma should be considered as a possible cause of gingival hyperplasia when the lesion does not respond to conventional treatment and the morphology is atypical.