A Case of Polychondritis in a Patient with Behçet's Disease
Article information
Abstract
Polychondritis is an inflammatory disorder that affects various catilagenous structures, and the clinical features include auricular, nasal and respiratory tract chondritis. It also involves the eyes, audiovestibular apparatus, joints and vascular structures. Polychondritis can be associated with several rheumatologic diseases such as systemic lupus erythematosus, rheumatoid arthritis and systemic vasculitis. However, polychondritis is a rare complication of Behçet's disease (BD) and only ten cases with combined BD and polychondritis have been reported on around the world. In this report, we describe a 40-year-old Korean man with BD who suffered from polychondritis that manifested as bilateral auricular chondritis, conjunctivitis and arthritis.
INTRODUCTION
Behçet's disease (BD) is a chronic inflammatory disease that is characterized by recurrent oral and genital ulceration and uveitis1), the other clinical manifestations include skin lesions, arthritis, thrombosis, neurologic symptoms and intestinal ulceration. Polychondritis is known as a rare complication of BD and no case of BD combined with polychondritis had been reported on until Firestein et al. reported on five patients with both BD and relapsing polychondritis (RP) in 19852). Only five more cases have been reported since then3-7). We recently experienced a case of a 40-year-old male patient with both BD and polychondritis, and we report herein on this unusual case along with a review of the literature.
CASE REPORT
A 40-year-old man was referred to our hospital for further evaluation of his fever, oral ulcers and skin rashes that had lasted for 10 days. He had previously suffered from intermittent oral ulceration and uveitis that had been treated with oral prednisolone and intraocular injections of triamcinolone at another hospital 1 year prior to this admission.
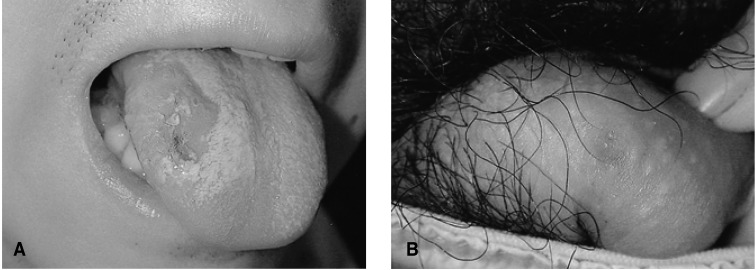
On the physical examination, his blood pressure was 130/80 mmHg, the pulse rate was 80 per minute and the body temperature was 38.4℃. He had conjunctival injections in both eyes and several aphthous ulcers on the tongue (Figure 1A) and buccal mucosa. A small round ulcer with tenderness was found on the penile root (Figure 1B). Several round erythematous skin lesions were present on the chest and thigh (Figure 2A). His left knee joint was swollen (Figure 2B) and tender, and needle aspiration revealed turbid inflammatory synovial fluid.
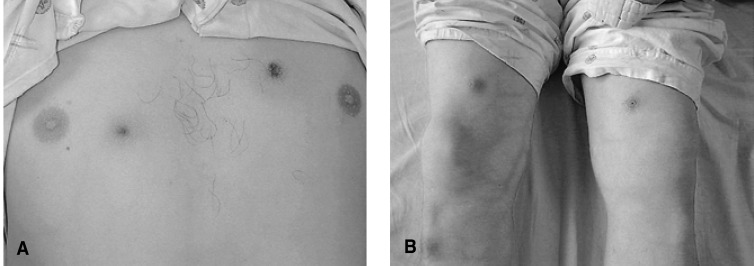
Several round erythematous skin lesions are noted on the chest wall (A) and thigh (B). Swelling of the left knee joint is also seen (B).
The laboratory findings were as follows: hemoglobin 14.8 g/dL, white blood cell count 10,400/µL, platelet count 291,000/µL, erythrocyte sedimentation rate 104 mm/hr and C-reactive protein 205 mg/L (normal range, 0~3.4 mg/L). The renal and liver function tests were normal and the tests for rheumatoid factor and antinuclear antibody were negative. The urinalysis was normal and no microorganisms were grown on both the blood and urine cultures. His chest radiography showed no abnormal findings and mild splenomegaly was observed on abdominal sonography. Gastroduodenoscopy revealed a gastric ulcer and no ileocecal lesion was found on colonoscopy examination. Skin biopsy was performed and the histologic examination showed perivascular mononuclear cell infiltrations, extravasation of the red blood cells and fibrinoid necrosis of the blood vessel walls; all of these findings were compatible with cutaneous vasculitis combined with BD (Figure 3).
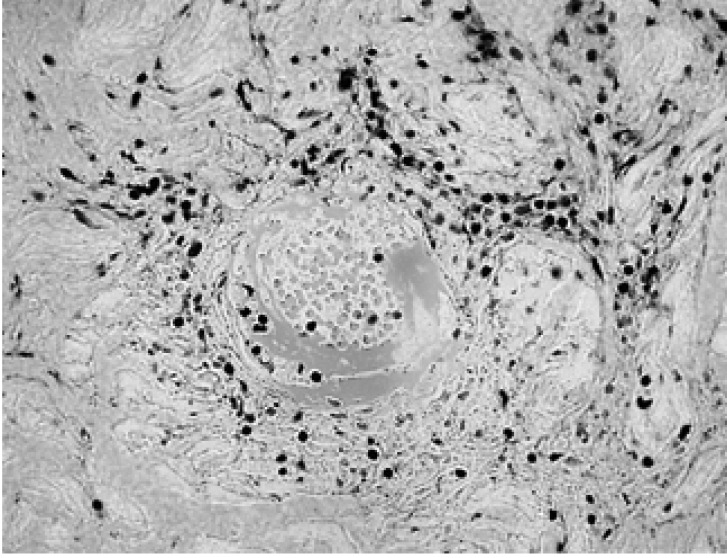
Photomicrograph shows the perivascular mononuclear cell infiltration, extravasation of the red blood cells and fibrinoid necrosis of the vessel wall (H&E stain, ×400).
On the 5th day of admission, both ear auricles became swollen, red and tender (Figure 4). He remembered having a similar auricular inflammation 2 years ago that had improved after taking non-steroidal anti-inflammatory drugs. After he was administered with colchicine 1.2 mg/day and prednisolone 30 mg/day, his clinical symptoms improved and he was discharged on the tenth day of admission.
DISCUSSION
Polychondritis is an inflammation of the various catilagenous structures such as the auricle, nose and trachea, and it has also been known to involve the eye and the joints. When polychondritis is relapsing, it is called relapsing polychondritis (RP)8). In RP, various cutaneous manifestations are also seen, including palpable purura, erythema nodosum, panniculitis, livedo reticularis and urticaria.
There have been case reports on polychondritis combined with other rheumatologic diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and systemic vasculitis9-11). However, there have been no reports of polychondritis combined with BD until Firestein et al. first reported on five patients who had the features characteristic of both BD and RP, and those authors termed this as mouth and genital ulcers with inflamed cartilage (MAGIC) syndrome2). Polychondritis is still known as a rare complication of BD and only five more cases of BD combined with polychondritis have been reported on since Firestein's report3-7).
In our patient, BD was diagnosed based upon the recurrent oral and genital ulcers, the skin vasculitis, the arthritis and the past history of uveitis. The chondritis developed at the bilateral auricles, eye and the joints during the acute exacerbation period of BD, and this resolved with the improvement of the symptoms of BD. This suggests that the development of polychondritis may have been associated with BD in our case. As for treatment for the combined cases of BD and RP, colchicine, steroid and dapsone have been reported to be effective3-7). Our patient also showed a good response to colchicine and oral prednisolone.
Skin involvement in BD includes various lesions such as erythema nodosum, pustules, papules, pseudofolliculitis, acneiform folliculits, Sweet's syndrome, or pyoderma gangrenosum12). The histopathology of the papulopustular lesions usually shows vasculopathy that is characterized by the perivascular infiltration of mononuclear cells, and this infiltrate is mainly composed of lymphocytes. The lesions are sometimes accompanied by luminal thrombosis, necrosis or mural fibrin deposition. Neutrophilic vascular reaction has also been reported13). The erythema nodosum-like lesion is known to be characterized by extravascular neutrophilic infiltration and panniculitis, and this can be followed by lymphocyte infiltration during the evolution of the skin lesions14). In our case, the main histopathylogic finding of the skin lesions was perivascular mononuclear cell infiltration and fibrinoid necrosis of the vessel walls, which are the characteristic findings of vasculopathy.
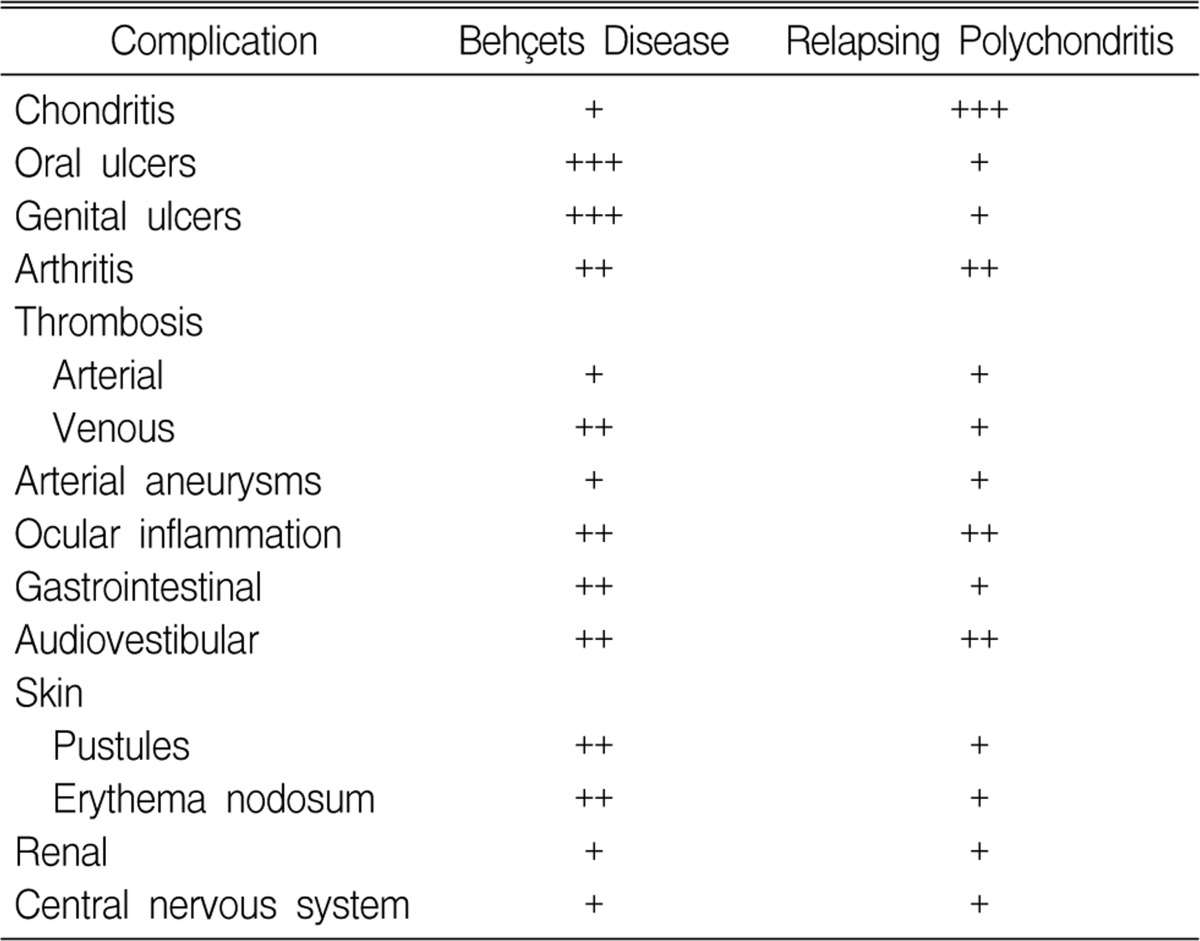
Although cases of BD combined with polychondritis have rarely been reported, BD and polychondritis do not seem to be separate entities. There are some overlapping manifestations for BD and RP. Firestein et al. compared the frequency of the various clinical features in BD and RP (Table 1), and they showed that some of the manifestations that are considered unique for one disease could be found in the other disease2). These similarities between the clinical manifestations of BD and RP imply that there seems to be something in common for the pathogenesis of BD and polychondritis. The pathogenesis of both BD and RP are associated with autoimmunity. In patients with RP, the antibodies to collagen type II, IX and XI are elevated in the sera15, 16) and the frequency of human leukocyte antigen (HLA)-DR4 is increased17). Additionally, RP has been associated with other autoimmune diseases such as RA and SLE9, 10). In patients with BD, autoantibodies such as antiendothelial cell antibody and antibody to α-tropomyosin have been found18-20) and it has been postulated that cross-reactivity and molecular mimicry between the peptides from the streptococcal proteins or the viral heat shock proteins (HSPs), which are homologues of human HSPs, and the mucosal antigens result in the selection of autoreactive T cells21). Firestein et al. have proposed that autoimmunity to components of the cartilage other than type II collagen, such as proteoglycans or elastic tissue, could be the common mechanism of BD and RP2). In particular, elastic tissue is ubiquitous in the human mucosa that is mainly involved in BD, and in the cartilage too, which is the major target of RP. However, because of the rarity of the combined cases, there have not yet been any investigations about the common pathogenesis of the two diseases, and so additional research is clearly needed. Although there have been only a few reports about polychondritis combined with BD, we think this combination of diseases might have been overlooked so far and closer scrutiny would reveal more cases.