Diffuse Alveolar Hemorrhage Associated with Antineutrophil Cytoplasmic Antibody levels in a Pregnant Woman Taking Propylthiouracil
Article information
Abstract
Propylthiouracil (PTU) is known to be a potential cause of antineutrophil cytoplasmic antibody (ANCA) positive small vessel vasculitis, resulting in glomerulonephritis and diffuse alveolar hemorrhage (DAH). Herein, we describe a 25-year-old pregnant woman who developed a perinulcear ANCA (p-ANCA) and myeloperoxidase ANCA (MPO-ANCA) positive DAH during PTU therapy. The patient improved after corticosteroid therapy and discontinuation of the PTU. Methimazole was prescribed in spite of the risk of recurrence of DAH because of the pregnancy. The patient is currently free from pulmonary problems. Our case shows that the alternative agent, methimazole, can be used to treat hyperthyroidism in a pregnant patient with PTU associated DAH.
INTRODUCTION
Diffuse alveolar hemorrhage (DAH) is a relatively rare, but potentially life-threatening complication of a wide variety of disorders. Most cases of DAH are associated with systemic vasculitis such as Wegener's granulomatosis, Churg-Strauss syndrome, microscopic polyangiitis, systemic lupus erythematosus, Goodpasture syndrome, or drug ingestion1, 2). Propylthiouracil (PTU) is a drug commonly used to treat hyperthyroidism. Recently PTU has been identified as a possible cause of antineutrophil cytoplasmic antibody (ANCA) positive small vessel vasculitis, resulting in glomerulonephritis and DAH3-5). We describe a case of perinulcear ANCA (p-ANCA) and myeloperoxidase ANCA (MPO-ANCA) positive DAH in a pregnant woman during PTU therapy.
CASE REPORT
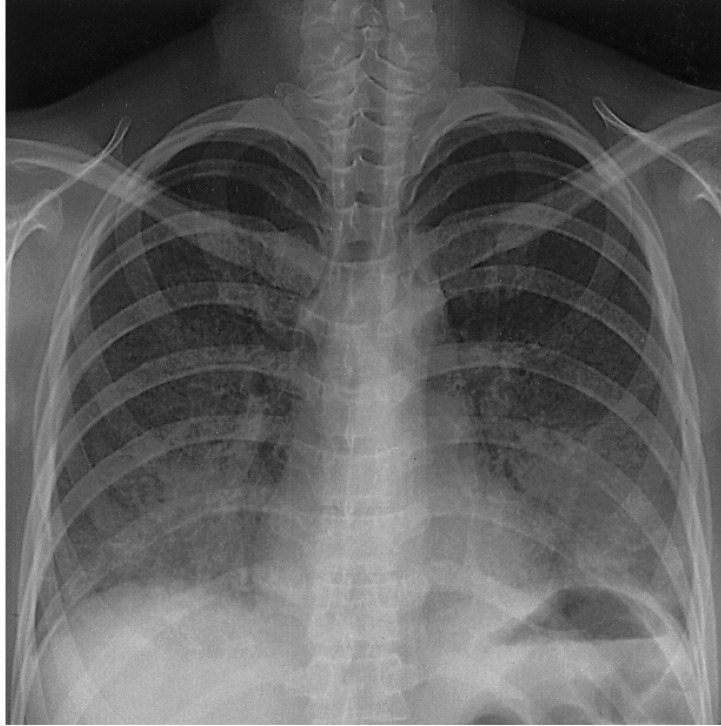
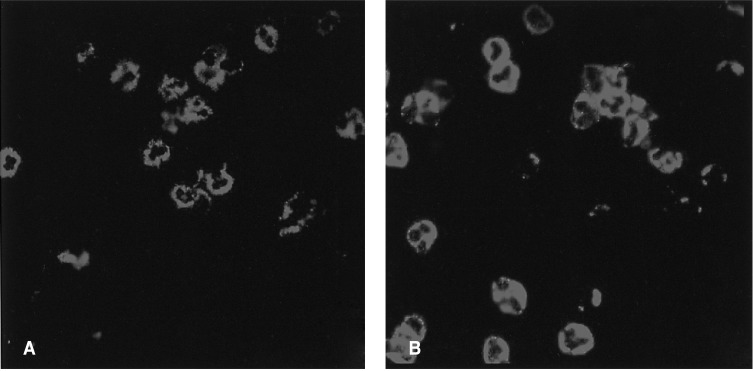
A 25-year-old woman in her first trimester of pregnancy was admitted to our hospital because of dyspnea and cough that developed three days prior to admission. The patient had a history of hyperthyroidism, which had developed at 16 years of age. She had been taking PTU for five years and was euthyroid. One month prior to admission PTU was resumed due to hyperthyroidism. On admission the temperature was 37.8℃, the blood pressure 120/70 mmHg, the pulse 110 beats per minute and the respiration rate 24 breaths per minute. The oxygen saturation was 96 percent while she was breathing ambient air. On physical examination, a few crackles were heard bilaterally and the thyroid gland was enlarged. Laboratory values showed a leukocyte count of 13,620/mm3, hemoglobin of 10.1 g/dL, platelet of 21,400/mm3 and a CRP of 1.27 mg/dL. Renal and liver function tests were normal. The prothrombin time was 11.2 seconds and the activated partial-thromboplastin time was 20.7 seconds. The T3 was 2.62 ng/mL (normal range, 0.79-1.49 ng/mL), free T4 0.42 ng/dL (normal range, 0.71-1.48 ng/dL) and thyroid stimulating hormone 0.004 IU/mL (normal range, 0.35-4.93 IU/mL). Urinalysis showed three to four red cells per high power field without proteinuria. A chest X-ray after abdominal protection showed bilateral heterogeneous pulmonary opacities (Figure 1). The patient was diagnosed as having pneumonia and was treated with ceftriaxone and azithromycin. On the third day of hospitalization, the patient rapidly deteriorated. She was transferred to the ICU. Mechanical ventilatory support was initiated for hypoxic respiratory failure. The CT scan showed multifocal areas of ground-glass opacities and consolidation in both lung fields (Figure 2). A surgical lung biopsy was considered but was not performed because of the risk of thyrotoxicosis. A bronchoscopy was performed and revealed active bleeding from segmental bronchi in the right and left lobes of the lung. The hemoglobin dropped to 7.3 g/dL and the serum p-ANCA was a strong positive and MPO-ANCA was positive with a titer of 255 AAU/mL (reference laboratory range, positive if 180 > AAU/mL; Figure 3). Tests for antinuclear antibody, anti-DNA antibody, rheumatoid factor, anti-glomerular-basement-membrane antibody, anti-proteinase 3 ANCA (PR3-ANCA), hepatitis serology and HIV were all negative. All additional biochemical testing was within normal limits. The PTU was discontinued and high dose intravenous corticosteroid therapy (methyprednisolone 125 mg every 6 hour) was started. Because of the pregnancy, methimazole was substituted for PTU. After initiation of corticosteroid therapy, the patient rapidly improved. Corticosteroid therapy was tapered slowly over four weeks, and the patient was discharged after one month. She remains in clinical remission six months later and the level of the MPO-ANCA has decreased to 159 AAU/mL.
DISCUSSION
PTU has recently been identified as a possible cause of ANCA positive small vessel vasculitis, resulting in abnormalities including glomerulonephritis, alveolar hemorrhage, and variable skin lesions. ANCA positive vasculitis in association with antithyroid drugs was first reported in 1993 by Dolman et al6). Since this initial report, the majority of cases have occurred in association with PTU treatment7).
Development of DAH associated with vasculitis is relatively rare yet frequently life-threatening and therefore requires prompt treatment. Testing for ANCA is useful in differentiation of the diseases commonly associated with alveolar hemorrhage. ANCAs are specific for antigens in neutrophil granules and monocyte lysosomes. They can be detected with indirect immuno-fluorescence microscopy by using alcohol-fixed neutrophils as substrates. Anti-PR3 antibodies produce cytoplasmic fluorescence and are found in 50 to 95 percent of cases of Wegener's granulomatosis. Anti-MPO antibodies produce perinuclear fluorescence and are found in more than 75 percent of microscopic polyangiitis and in a high proportion of drug-induced vasculitis, particularly following exposure to hydralazine or PTU2, 8). Other serologic markers such as anti-GBM antibody, antinuclear antibody, double-stranded DNA antibody and antiphospholipid antibody are also useful for the diagnosis of DAH.
Our patient had no medical history of bleeding diasthesis, toxic exposure, disease of the upper respiratory tract including oropharynx, nose and sinuses or asthma. There were no constitutional symptoms such as anorexia, weight loss or fatigue. There were no signs of joint, ocular or skin involvement that would suggest the presence of a collagen vascular disease. Although MPO-ANCA is frequently associated with microscopic polyangiitis, it was possible to exclude microscopic polyangiitis as the cause for of DAH in this case. This is because most cases of microscopic polyangiitis have rapidly progressive glomerulonephritis in the early stage of disease. In addition, after the discontinuation of PTU, the titer of MPO-ANCA was markedly decreased and other systemic manifestations commonly associated with vasculitis were not observed in the absence of corticosteroid maintenance therapy. The presence of microscopic hematuria, on admission, suggested glomeronephritis, but at a follow-up examination, the hematuria was resolved. Although we cannot definitively prove that PTU was responsible for the ANCA positive vasculitis, the clinical course of the patient's illness suggests the association of PTU and the ANCA positive DAH. Untreated pregnant women with hyperthyroidism are at risk for preeclampsia, heart failure and impaired fetal outcome9). Therefore, we replaced PTU with methimazole and the patient did well with no further complications.
The mechanism of PTU induced ANCA positive vasculitis remains unclear. It has been suggested that antithyroid drugs or metabolites may cross-react with neutrophilic cytoplasmic antigens. Lee et al. suggested that PTU interacts with MPO to change the heme structure of the enzyme, which may then act as a hapten and promote formation of autoantibodies in susceptible individuals10). However, Von Schmiedeberg et al. speculated that, in the presence of MPO, PTU is converted to PTU sulfonate, which is immunogenic for T cells, and these T cells in turn activate B cells, which mediate vascular injury11).
In conclusion, this case highlights that DAH should be considered as a potential side-effect of PTU when ANCA positive vasculitis during PTU therapy develops; an alternative agent, such as methimazole, is an option for treating underlying hyperthyroidism in pregnant patients.