Marked Recovery From Paraquat-Induced Lung Injury During Long-Term Follow-up
Article information
Abstract
Background/Aims
Paraquat-induced lung injury has been considered a progressive and irreversible disease. The purpose of this study was to report the long-term evolution of lung lesions in eight survivors with significant paraquat-induced lung injuries who could be followed-up for longer than 6 months.
Methods
We retrospectively examined high-resolution computed tomography and pulmonary function test of eight survivors with significant paraquat-induced lung injurys.
Results
High-resolution computed tomography revealed a predominant pattern of irregularly shaped consolidation with traction bronchiectasis at 1-2 months after paraquat poisoning, a mixed pattern of irregularly shaped consolidation and ground-glass opacity at 3-12 months, and a mixed pattern of consolidation, ground-glass opacity, and honeycombing at 1-2 years. At 3-12 months after paraquat ingestion, the areas of consolidation had markedly decreased and the decreased lung volume had returned to normal. At 1-2 years after paraquat poisoning, the cystic changes had disappeared. At 2-3 years after paraquat poisoning, the decrease in forced vital capacity had greatly improved to the normal range.
Conclusions
Recovery of nearly normal pulmonary structure and function may occur over several years following paraquat poisoning. Pulmonary function (both forced vital capacity and forced expiratory volume in 1 sec) evolved toward normal in the long-term survivors of paraquat poisoning with initial prominent lung injuries.
INTRODUCTION
Paraquat (1,1'-dimethyl-4,4'-bipyridylium dichloride) is notorious throughout the world as a potent human poison. Death typically occurs as a result of progressive and irreversible pulmonary fibrosis in 5-31 days after poisoning [1]. Pulmonary involvement has been considered indicative of a relentlessly progressive disease with no hope of reversal [2,3]. However, some survivors with lung injury after paraquat poisoning have been recently reported [4,5], so elucidating the evolution of paraquat-induced lung injury has become necessary.
High-resolution computed tomography (HRCT) is an excellent tool for detecting paraquat-induced lung injury [6,7]. We have previously described HRCT findings of lungs in patients less than 1 months after paraquat poisoning [6]. The predominant finding within the first 7 days is areas of ground-glass attenuation that change into areas of consolidation associated with bronchiectasis and areas of irregular lines on follow-up HRCT scans [6].
The present study was conducted to observe the evolution of pulmonary injury in long-term survivors after paraquat intoxication by serial follow-up using HRCT and pulmonary function tests.
METHODS
Study subjects
We retrospectively examined the medical records of 1,189 Korean patients with acute paraquat poisoning who were admitted to the Institute of Pesticide Poisoning at Soonchunhyang University Cheonan Hospital (SCH, Cheonan, Korea; a specialized institute for pesticide poisoning in Korea) from 1995 to 2001. An initial 682 HRCT scans were obtained 6-8 days (mean, 7 days) after paraquat poisoning in all 682 patients who survived to 7 days after paraquat exposure. Among them, 191 patients with prominent pulmonary lesions on HRCT were monitored weekly or monthly with HRCT until the findings were constant. Follow-up HRCT scans became stationary before 6 months after paraquat poisoning in most of the patients.
Only eight patients included in the study had abnormal pulmonary findings on their initial HRCT scan and had prominent evolutional changes of the initial findings on follow-up HRCT scans more than 6 months after paraquat poisoning: they underwent the last follow-up HRCT scanning 15-118 months after paraquat poisoning. All HRCT scans were obtained with one of two scanners (CT-W 700; Hitachi Medical, Tokyo, Japan; or a GE Prospeed; General Electric Medical Systems, Milwaukee, WI, USA). The HRCT images were reviewed separately by three experts in paraquat-induced lung lesions and decisions were reached by consensus. The HRCT findings were evaluated by duration after paraquat exposure and categorized into five periods: 1-2 months, 3-12 months, 1-2 years, 2-3 years, and 3-8 years. We also observed the changes in HRCT findings on serial follow-up HRCT scans in the same patients. The HRCT findings were evaluated by the existence of ground-glass opacity, pulmonary consoli-dation, bronchiectasis, air trapping, and subpleural lines.
Standardized medical emergency procedures were conducted according to the Treatment Guideline for Paraquat Poisoning published by SCH [8]. Briefly, gastric lavage was performed on all subjects seen within 2 hours after ingestion, and 100 g of Fuller's earth in 200 mL of 20% mannitol was given if intoxication had occurred within the previous 12 hours. Extracorporeal removal was performed if a urinary paraquat test was positive. All of these procedures were conducted after obtaining informed consent. The paraquat exposure was assessed based on the amount of paraquat ingested as reported by the subject and on a semiquantitative measure of urine paraquat [9]. Clinical parameters of PaO2, creatinine, aspartate aminotransferase, alanine aminotransferase, and amylase were obtained at the time of the SCH visit and during the follow-up period. Hepatic dysfunction was defined as either aspartate aminotransferase or alanine aminotran-sferase >80 IU/L (double the normal upper limits), renal dysfunction was defined as serum creatinine >2.0 mg/dL, and pancreatic dysfunction was defined as blood amylase >320 IU/L (double the normal upper limit) based on the initial or follow-up laboratory data. Pulmonary function tests were performed in seven patients, with both initial and follow-up tests being performed in five of these patients. This study was approved by the institutional boards at SCH.
Statistical analysis
Data are presented as the median and range for continuous variables and frequency (%) for categorical variables. We analyzed differences among the time points in absolute value for the pulmonary function test measures with the nonparametric Kruskal-Wallis test. A probability value of p<0.05 was considered statistically significant, with all statistical analyses performed using SPSS for Windows (version 10.0; SPSS Inc., Chicago, IL, USA).
RESULTS
The general characteristics of the patients are summarized in Table 1. Their median age was 35 (18-66) years, and the male-to-female ratio was 3:5. Three patients (cases 2, 3, and 6) had unintentionally ingested a paraquat solution that was in an unlabeled bottle, and the other five cases were ingested intentionally. All patients had consumed a 24.5% concentrate of paraquat. All patients were transferred from other medical facilities to SCH. The median estimated amount of paraquat dichloride ingested was 20 (20-120) mL. At SCH, urinary paraquat was detected in six patients at 42 (2-128) hours after paraquat ingestion. The average hospital stay was 29 (11-72) days. None of the patients smoked cigarettes or had a history of pulmonary disease. During the admission and follow-up period, liver dysfunction was noted in 62.5% of patients. All patients had renal dysfunction, but only one patient had pancreatic dysfunction. Five patients experienced severe hypoxia (PaO2 <60 mmHg) during admission. The mean follow-up duration was 39 96 The Korean Journal of Internal Medicine Vol. 24, No. 2, June 2009 (21-104) months, and all patients were alive at the end of December 2003.
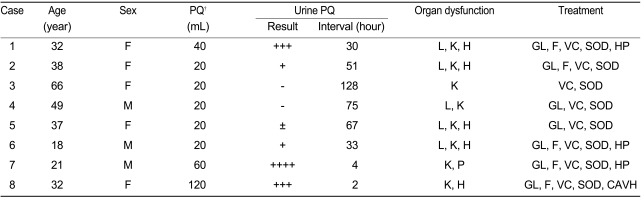
Forty-three HRCT scans of the lungs in eight patients with paraquat poisoning were reviewed. Ground-glass opacity was observed in all patients 1 week after paraquat ingestion (Fig. 1A, 2A and 3A). The patterns of 20 HRCT scans of lungs obtained at 4 weeks after paraquat poisoning were evaluated (Table 2). The areas of ground-glass opacity had changed to an irregularly shaped consolidation with traction bronchiectasis at 1-2 months (Fig. 4A; Table 2). A mixed pattern of the irregularly shaped consolidation and ground-glass opacity was observed at 3-12 months in all patients (Fig. 1B, 2B and 4B; Table 2). At 1-2 years after paraquat poisoning, irregularly shaped ground-glass opacity with consolidations was observed in four patients and honeycombing was seen in two patients (Fig. 3B and 5B; Table 2).
Representative HRCT findings in an 18-year-old man (case 6). (A) HRCT scan obtained 1 week after paraquat ingestion shows diffuse ground-glass opacity in both upper lobes. (B) HRCT scan obtained 5 months after paraquat ingestion shows irregularly shaped air-space consolidation in both upper lobes. Air spaces (3 cm) are seen in the anterior periphery of the left upper lobe. (C) HRCT scan obtained 15 months after paraquat ingestion shows irregularly shaped consolidation in the left upper lobe in the absence of air trapping. Focal consolidation in the right upper lobe of (A) is not evident.

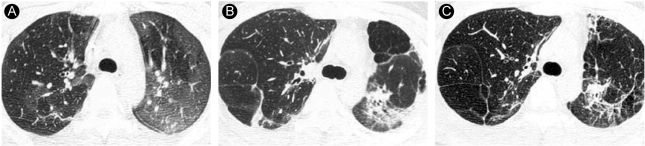
Representative HRCT findings in a 32-year-old woman (case 8). (A) HRCT obtained 1wk after paraquat ingestion shows ill-defined diffuse ground-glass opacity in both lungs. (B) HRCT scan obtained 6 months after paraquat ingestion shows air-space consolidations with small cystic changes and traction bronchiectasis in the decreased left lung. Focal consolidation is seen in the anterior periphery of the right middle lobe. Subpleural lines are seen in the posterior periphery of the right upper lobe. (C) HRCT scan obtained 27 months after paraquat ingestion shows localized honeycombing in the left lung. Irregularly shaped consolidations in the left lung on (B) have decreased markedly. (D) HRCT scan obtained 7 years after paraquat ingestion shows multifocal honeycombing.
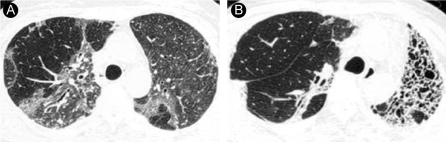
Representative HRCT findings in a 38-year-old woman (case 2). (A) HRCT scan obtained 1 week after paraquat ingestion shows diffuse ground-glass opacity in both lungs. (B) HRCT scan obtained 15 months after paraquat ingestion shows focal consolidation in the right upper lobe and honeycombing in the left upper lobe.

Follow-up high-resolution computed tomography (HRCT) patterns according to the time after paraquat poisoning in eight long-term survivors of prominent paraquat-induced lung injuries

Representative HRCT findings in a 32-year-old woman (case 1). (A) HRCT scan obtained 1 month after paraquat ingestion shows irregularly shaped multifocal air-space consolidation with traction bronchiectasis in both lower lobes. (B) HRCT scan obtained 11 months after paraquat ingestion at the same level as in (A) shows that irregularly shaped multifocal air-space consolidations have changed to irregularly shaped multifocal ground-glass opacities. However, the extent of the lesions had not changed.
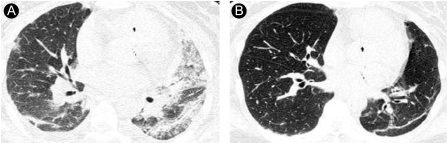
Representative HRCT findings in a 37-year-old woman (case 5). (A) HRCT scan obtained 2 weeks after paraquat ingestion shows diffuse ground-glass opacity in the left lung and focal ground-glass opacity in the right lung. (B) HRCT scan obtained 20 months after paraquat ingestion shows subpleural lines in the posterior portion of the left upper lobe, but bilateral consolidation is not evident.
The areas of consolidation on follow-up HRCT scans at 3 months after paraquat poisoning had markedly decreased relative to that at 1-2 months (Fig. 4 and 5). The small cystic changes with consolidation and the decrease in lung volume at 3-12 months changed to honeycombing at 1 year after paraquat poisoning; the cystic changes disappeared and the lung volumes increased at 7-8 years after paraquat poisoning (Fig. 2). The extent of the lung lesions remained unchanged in the majority of cases, but the irregular consolidation, especially focal involvement, had disappeared (Fig. 4 and 5). Small cystic changes and focal air trapping were observed transiently at 1-12 months (Fig. 1 and 2). Subpleural lines were also observed 3 months to 8 years after paraquat poisoning (Table 2).
In pulmonary function tests, the forced vital capacity (FVC) and forced expiratory volume at 1 sec (FEV1) decreased markedly, and the FEV1/FVC ratio was normal at 1 month after paraquat poisoning. FVC at more than 2-3 years after paraquat poisoning had significantly improved (p=0.047, Fig. 6A), but not FEV1 (p=0.327, Fig. 6B).
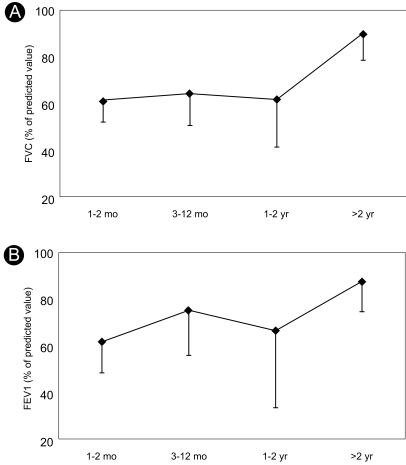
Pulmonary function test results according to the time after paraquat poisoning in long-term survivors of prominent paraquat-induced lung injuries. (A) FVC, forced vital capacity, *p=0.047; (B) FEV1, forced expiratory volume in 1 sec, p=0.327. Statistical analyses were performed with the nonparametric Kruskal-Wallis test.
DISCUSSION
Paraquat poisoning is a significant cause of mortality worldwide, and intentional self-poisoning with paraquat is a major public health issue in some countries such as Korea [10]. The main cause of death of acute paraquat poisoning after 1 week is known to be respiratory dysfunction due to lung damage. In adults, ingestion of paraquat at 25-35 mg/kg body weight may produce survivable restrictive pulmonary lesions [11]. Survivors of paraquat poisoning are left with a permanent restrictive-type of pulmonary dysfunction, but a long-term follow-up of lung function has indicated recovery of pulmonary function in survivors of paraquat poisoning [12].
Little is known regarding long-term changes in lung structure and function in survivors of acute paraquat poisoning. The present study is the first serial follow-up of the structure and function of the lung that has documented the long-term course of pulmonary damage after acute paraquat poisoning.
HRCT findings in lungs less than 1 month after paraquat poisoning have already been described [6]. Our findings were similar in that the initial areas of ground-glass attenuation within the first 7 days changed into areas of consolidation associated with bronchiectasis on follow-up HRCT scans [6] less than 1 month after paraquat poisoning. However, our HRCT findings from more than 1 months after paraquat poisoning and serial follow-up are the first to be reported. A surprising finding in the present study was that the consolidations had markedly decreased, the cystic changes had disappeared, and the decreased lung volume had returned to normal at 3-12 months after paraquat ingestion. The extent of paraquat-induced lung lesions remained unchanged in the majority of cases, but the irregular consolidations, especially focal involvement, had disappeared. The transient HRCT findings after paraquat ingestion were small cysts, focal air trapping, and subpleural lines. The small cysts were observed in two cases at 1-12 months after paraquat exposure, and focal air trapping was observed in one case at 3-12 months. Im et al [7] observed small cysts and linear opacity at 2-4 weeks and focal honeycombing at 4 weeks on simple chest X-rays.
The FVCs of our patients with severe paraquat poisoning dramatically and significantly recovered after 2-3 years. The lag time to recovery was longer than in a previous case study [13], but this difference may have been attributable to more severe paraquat-induced lung dysfunction in our cases.
Based on the long-term follow-ups of the cases presented here, recovery of normal or nearly normal pulmonary structure and function is possible over several years. When considering possible mechanisms for this unanticipated pulmonary recovery, the most likely hypothesis is that an inflammatory factor is present during the acute phase of the poisoning, predisposing one to the early onset of pulmonary fibrosis, which may improve over time [14].
Our study had several limitations, although we do not believe that they invalidate its conclusions. First, we did not measure plasma paraquat levels in all cases, which may have more accurately reflected the severity of paraquat exposure. However, we and others observed the significant relationship between plasma paraquat concentration and urine paraquat concentration employed in the present study [15,16]. Second, we could not correlate HRCT and pathology due to serial examination of the lung pathology. In a previous study, we observed interstitial fibrosis and mononuclear cell infiltration on lung tissue and ground-glass opacity in patients 1 weeks after paraquat exposure [6]. The complete resolution of the ground-glass opacity on HRCT over time has been reported in amiodarone-induced lung disease [17]. Therefore, we believe the areas of consolidation with bronchiectasis on HRCT scans obtained at 1-2 months revert to normal or near-normal structure.
In conclusion, typical long-term HRCT findings of the lung after paraquat poisoning were irregularly shaped pulmonary consolidation at 1-2 months after paraquat exposure, and mixed patterns of irregularly shaped pulmonary consolidation and ground-glass opacity or honeycombing at 3-12 months. The attenuation of the pulmonary consolidation began in all patients at 3-12 months and the focal pulmonary consolidation may have disappeared during this period. Therefore, progressive pulmonary fibrosis was not seen in long-term survivors after acute paraquat poisoning in the present study. Our findings suggest that paraquat-induced lung injury may not be irreversible or progressive. Not only did lung volume increase, lung function also improved over time in long-term survivors of paraquat poisoning with prominent lung injuries.