Natural History and Renal Pathology in Patients with Isolated Microscopic Hematuria
Article information
Abstract
Background/Aims
No definite conclusions have been reached about the natural history of patients with isolated microscopic hematuria (IMH). In this study, we observed the natural history of patients with IMH and examined factors related to a pathologic diagnosis and subsequent prognosis.
Methods
We retrospectively evaluated 156 subjects with IMH who had a renal biopsy performed. Of the 156 subjects, 33.3% were diagnosed with IgA nephropathy, 23.7% with mesangial proliferative glomerulonephritis, 15.4% with glomerular minor lesion, and 12.8% with thin basement membrane nephropathy; 6.4% had normal biopsies.
Results
We followed up with 100 subjects for about 31 months. During this follow-up period, two subjects who had received a pathologic diagnosis of IgA nephropathy developed chronic kidney disease. During the course of the study, one of these subjects presented with proteinuria and hypertension and the other with proteinuria. The overall incidences of proteinuria and hypertension were 6% and 5% respectively.
Conclusions
The prognosis for patients with IMH was relatively favorable, but patients developing proteinuria and/or hypertension require careful observation and management during the follow-up period.
INTRODUCTION
Isolated microscopic hematuria (IMH) is a common urinary abnormality in clinical practice [1-4]. IMH is defined as persistent asymptomatic microscopic hematuria without hypertension, proteinuria, renal insufficiency, urinary tract infection, or structural abnormality of the urinary tract, and may it be an early sign of glomerulonephritis [3-7]. No definite conclusions have been reached regarding the natural history of patients with IMH. Until recently, a prognosis of IMH was considered benign, but new studies have revealed that some patients have a poor outcome, particularly those who developed proteinuria and/or hypertension during the follow-up period [8-11]. The decision to perform a renal biopsy on a patient with IMH also remains controversial. Some nephrologists do not recommend a renal biopsy because identification of a specific disease will not make any difference to either patient management or treatment outcome; other nephrologists recommend a renal biopsy because they think it can provide a more accurate diagnosis and prognosis [12,13]. In this study, we retrospectively reviewed data about 156 patients with IMH who had undergone a percutaneous renal biopsy to observe the natural history of patients with IMH and to examine factors related to pathologic diagnosis and prognosis.
METHODS
Subjects
This study was retrospective and was conducted in accordance with the principles of the 1983 Helsinki Declaration. Potential subjects were patients who had been diagnosed with IMH and had undergone a percutaneous renal biopsy between June 2002 and August 2007. We reviewed the medical history and data of these patients at initial presentation. Patients were included in the study if they had persistent hematuria (red blood cell per high-power field in urinary sediment >5 on three or more occasions over 4 or more weeks, and sterile urine in a routine culture), serum creatinine <1.2 mg/dL, a urine protein to creatinine ratio <0.3 g/mg, resting systolic blood pressure <40 mmHg, and diastolic blood pressure <90 mmHg. We excluded patients who had a history of edema or gross hematuria or who were younger than 15 years. Computed tomography, abdomen sonography, and/or urine cytology were performed for all subjects, and we excluded patients with stones, malignancy, trauma, and/or tuberculosis in the urinary tract. We also excluded patients with any systemic disease or a family history suggestive of Alport's syndrome. A total of 156 subjects were enrolled in the study. In all subjects, the amount of proteinuria was measured by determining the urine protein-to-creatinine ratio. The glomerular filtration rate (GFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation. Renal biopsy specimens were examined by a renal pathologist using light microscopy, indirect immunofluorescence, and electric microscopy. The pathologic diagnosis was conducted according to WHO criteria for renal disease classification [14]. Of the 156 subjects, 100 were monitored through follow-up appointments for at least one year. These follow-up appointments also included testing for clinical parameters associated with hematuria, GFR, proteinuria, and hypertension. Chronic kidney disease (CKD) was defined as an estimated GFR <60 mL/minute/1.73 m2 for 3 months or more. All parameters were recorded at each clinic visit.
Statistical analysis
All results are expressed as mean±standard deviation. Data were compared using Student's t-test, the χ2 test, or Fisher's test, as appropriate. The Kaplan-Meier method with a log rank test was used to compare the cumulative incidence of survival free of CKD, proteinuria, and hypertension. The Cox proportional hazard model was used to determine an independent factor for risk of CKD, proteinuria, and hypertension. Variables included (at presentation) age, gender, body mass index, mean arterial pressure, degree of proteinuria, serum creatinine level, and pathology of the renal biopsy. A p values of <0.05 were considered significant. All statistical analyses were performed using SPSS version 11.5 (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline patient characteristics
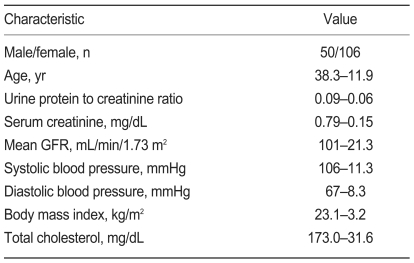
Table 1 list clinical characteristics for the 156 subjects with IMH at the time of the renal biopsy. Subjects had a mean age of 38.3±11.9 years and included 50 males and 106 females. Mean systolic blood pressure was 106±11.3 mmHg, and mean diastolic blood pressure was 67±8.3 mmHg. Mean serum creatinine concentration was 0.79±0.15 mg/dL, and mean estimated GFR was 101±21.3 mL/minute/1.73 m2, according to the abbreviated MDRD study equation. Mean urine protein-to-creatinine ratio was 0.09±0.06 g/mg.
Table 2 lists the pathologic diagnoses for the 156 subjects with IMH. The most frequent diagnosis was IgA nephropathy (52 subjects, 33.3%). Subclasses of the Haas classification were distributed heterogeneously. Mesangial proliferative glomerulonephritis (MsPGN) was observed in 37 subjects (23.7%), minimal mesangiopathy in 24 subjects (15.4%), and thin basement membrane nephropathy (TBMN) in 20 subjects (12.8%). Ten subjects (6.4%) had a normal biopsy, eight (5.1%) had focal segmental glomerulosclerosis (FSGS), three (1.9%) had membranous glomerulonephritis (MGN), and two (1.3%) had membranoproliferative glomeruolonephritis (MPGN).
Clinical course
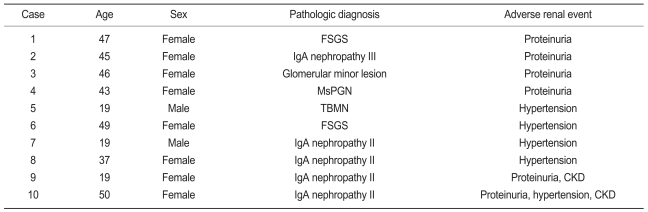
Of the 156 subjects, 100 were monitored through follow-up appointments for at least 1 year. These 100 subjects included 22 males and 78 females with a mean age of 35.9±11.4. The mean follow-up duration from renal biopsy to the last outpatient check-up was 31.6±14.1 months. A comparison of clinical data from all 156 subjects and the subset of 100 subjects revealed no statistical differences. Table 3 lists the clinical findings for the 100 subjects who participated from the renal biopsy through to the end of the follow-up period. Mean systolic blood pressure increased significantly (p<0.001) through this follow-up period, but no significant changes appeared in mean diastolic pressure, mean urine protein-to-creatinine ratio, mean serum creatinine concentration, or mean GFR between the time of initial presentation and the end of the follow-up period. Table 4 lists the characteristics of subjects who developed CKD, proteinuria, and hypertension during the follow-up period. By the end of the follow-up period, two subjects (2%) had developed CKD. No subjects had reached end-stage renal disease by this point. Two subjects developed CKD during the follow-up period; one of these developed proteinuria and hypertension, and the other developed only proteinuria. Pathologic diagnoses of the two subjects who developed CKD revealed IgA nephropathy. During the follow-up period, proteinuria appeared in six subjects (6%). The histological findings for these subjects were IgA nephropathy (three cases), FSGS (one case), MsPGN (one case), and minimal mesangiopathy (one case). Two of the six subjects who presented with proteinuria progressed to CKD. During the follow-up period, hypertension appeared in five subjects (5%). The pathologic diagnoses of these subjects were IgA nephropathy (three cases), FSGS (one case), and TBMN (one case). One of the five subjects who presented with hypertension progressed to CKD. During the follow-up period, microscopic hematuria disappeared in four subjects (4%), and the pathologic diagnosis for all these patients was IgA nephropathy. None of these subjects presented with CKD, proteinuria, or hypertension during the follow-up period.
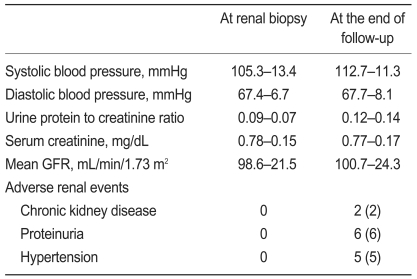
Comparison of clinical data in 100 patients with isolated microscopic hematuria during the follow-up period
We used a multivariate model (Cox proportional hazard model) to determine the independent risk factor for adverse renal events (CKD, proteinuria, or hypertension). We were unable to find a useful clinical parameter from data at presentation including age, gender, body mass index, mean arterial pressure, the degree of proteinuria, serum creatinine level, and pathology of the renal biopsy.
DISCUSSION
In this study, we investigated the pathologic diagnosis of 156 patients with IMH and the natural history of 100 of these patients who were monitored through follow-up appointments for a mean duration of about 36 months. Subjects with IMH had various diagnoses. The most common cause of IMH was glomerulonephritis, especially IgA nephropathy. The prevalences of IgA nephropathy and TBMN were 33.3% and 12.8% respectively, similar to previous reports [1-6]. Previous studies have reported that IgA nephropathy and TBMN are present in 10-60% of patients with IMH. Interestingly, the prevalence rate of IgA nephropathy and TBMN differed by geographic location because the frequency of urinalysis screening, indications for renal biopsy in patients with IMH, and availability of electron microscopy differs among countries [13-18]. We also documented the presence of relatively severe pathologic types, such as FSGS, MGN, and MPGN, in a number of cases, implying a clinico-pathological disassociation. Some, but not all, previous studies have also reported the presence of relatively severe pathologic types in subjects with IMH. This finding might be related to the number of subjects enrolled in the studies. Therefore, renal biopsy in patients with IMH might provide a more accurate diagnosis and also yield prognostic indicators including interstitial fibrosis and tubular atrophy [19,20].
No definitive prognosis is available for patients with IMH. Many previous studies have reported that the prognosis for patients with IMH is good, but some recent studies have reported poor outcomes. In particular, they found that subjects who developed proteinuria during the study, as well as subjects with developmental hypertension during the follow-up period, were more liable to have renal function deterioration than were those who had been diagnosed with proteinuria at the beginning of the study [8-11]. Of our subjects, two (2%) developed CKD during the mean 36 months of follow-up; the pathologic diagnoses for these subjects were both IgA nephropathy. The incidence of proteinuria and hypertension were 6% and 5% respectively during the follow-up period. Our data support that the prognosis of patients with IMH is not benign, and IMH might gradually progress to proteinuria and/or hypertension with time. One of the two subjects who developed CKD also developed proteinuria and hypertension during the follow-up period, whereas the other developed only proteinuria. Our data might also indicate that the development of proteinuria and/or hypertension during the follow-up period is a risk factor for progression to CKD in patients with IMH. Therefore, careful long-term follow-up and early detection of patients who show progression is important, and strategies for treatment should be designed early for these patients in an attempt to prevent CKD [4]. We found that during the follow-up period, hematuria disappeared in some subjects with asymptomatic persistent hematuria. Coppo and D'Amico [21]. also reported spontaneous remission or complete recovery 5-30% of adult patients with IgA nephropathy over a long-term follow-up period. In this study, hematuria disappeared in 4% of all subjects. The pathologic diagnoses for these subjects all included IgA nephropathy, and none developed CKD, proteinuria, or hypertension during the follow-up period. However, it is not clear whether clinical remission represents pathological remission. Alarmartine et al. [22] conducted repeated renal biopsies on patients with primary IgA nephropathy and did not observe pathological remission even in a patient with complete clinical remission. Therefore, careful long-term follow-up might be needed even for patients with IgA nephropathy whose hematuria disappears during the follow-up period.
Indications for renal biopsy in patients with asymptomatic persistent microscopic hematuria are still poorly defined. IMH seems to be a specific indication for a renal biopsy only among a minority of nephrologists; most nephrologists consider the prognosis for patients with IMH to be good and think that a renal biopsy will not affect treatment management or outcome [12,13]. However, Shu et al. highlighted the importance of performing a renal biopsy on IMH patients because minimal urinary abnormality with normal renal function at presentation does not necessarily imply a favorable outcome in certain patients; in addition, tubular atrophy and interstitial fibrosis, but not glomerular change, correlate with a worse prognosis [23]. Sparwasser et al. [24] suggested that a kidney biopsy be considered optional, and that once a diagnosis is established, repeated and unnecessary examinations can be avoided in young patients with persistent hematuria. Shen et al. [4] suggested that the urinary albumin/creatinine ratio, serum IgA level, and serum IgA/C3 ratio can serve as non-invasive markers to predict the necessity of a renal biopsy in adult patients with IMH. In this study, clinical findings at presentation were not helpful in differential diagnoses of patients with IMH. Renal biopsies provided a diagnosis for most IMH subjects and helped minimize unnecessary further investigation of their hematuria. Early pathologic diagnosis of patients with IMH helped to distinguish those with major glomerulonephritis, who had worse long-term prognoses than those with TBMN or a normal biopsy. These diagnoses enabled careful follow-up and consideration of early therapeutic strategies. However, renal biopsy results did not alter the management of patients with IMH at the time of presentation. Furthermore, it is not clear whether early diagnosis of IgA nephropathy has a prognostic impact, as these results can only be obtained after careful long-term follow-up. A renal biopsy is an invasive and costly procedure [25], although in our study the procedure did not result in major complications. Therefore, a renal biopsy may be optional for patients with IMH and indicators such as microalbumiuria may be a useful tool to aid in decisions about whether to proceed with a renal biopsy.
In conclusion, we found that glomerulonephritis was the major cause of IMH. The prognosis for patients with IMH may be favorable. However, careful observation and management are required for patients who develop proteinuria and/or hypertension during the follow-up period. A renal biopsy may be optional in consideration of its benefits and risk.