Elevation of Serum Ferritin is Associated with the Outcome of Patients with Newly Diagnosed Multiple Myeloma
Article information
Abstract
Background/Aims
Serum ferritin is a marker of acute phase reactions and iron storage. In addition, hematologic malignancies are associated with elevated serum ferritin levels. Other studies have suggested that ferritin is a surrogate for advanced disease and has an impact on relapse, because elevated serum ferritin predicts overall survival (OS) and relapse-free survival following autologous stem cell transplantation for lymphomas.
Methods
We studied 89 consecutive patients with newly diagnosed multiple myeloma to determine the value of serum ferritin in comparison with known prognostic factors.
Results
The OS in the elevated serum ferritin group (≥300 ng/mL) was shorter than that in the normal serum ferritin group (<300 ng/mL, p<0.001) after a median follow-up of 25 months. In univariate analysis, elevated ferritin was correlated with poor survival in the patients (relative risk [RR], 2.588; 95% confidence interval [CI], 1.536 to 4.358; p<0.001). Furthermore, multivariate analysis showed that elevated serum ferritin was an independent predictor of mortality in patients with multiple myeloma (RR, 2.594; 95% CI, 1.403 to 4.797; p=0.002).
Conclusions
The serum ferritin can a prognostic parameter of survival as well as disease activity in patients with multiple myeloma.
INTRODUCTION
Multiple myeloma (MM) is a plasma cell dyscrasia characterized by the slow proliferation of malignant plasma cells, primarily in the bone marrow. Several prognostic parameters are correlated with survival in patients with MM; these include β-2-microglobulin (B2MG), C-reactive protein (CRP), lactate dehydrogenase (LDH), the bone marrow plasma cell percentage (BMPC), albumin, creatinine, hemoglobin, performance status, and conventional cytogenetics [1-4].
Elevated levels of serum ferritin, the most widely used surrogate marker for iron storage, are associated with worse outcomes following transplantation for acute leukemia and myelodysplastic syndrome [5]. In addition, elevated pre-transplantation ferritin levels were associated with relapse following autologous hematopoietic stem cell transplantation for lymphoma [6]. A recent study reported that the serum ferritin level was increased in hematologic malignancies [7].
No study has examined whether the serum ferritin level reflects the outcome in MM. Therefore, we measured the serum ferritin levels in newly diagnosed MM patients to determine whether the level is correlated with outcome and is an independent predictor of survival in patients with MM.
MATERIALS AND METHODS
Patient population
Eighty-nine consecutive patients with MM seen at Pusan National University Hospital between 2000 and 2006 were evaluated retrospectively. There were 46 males and 43 females, and their median age was 64 years (range, 38 to 87). The median follow-up duration was 24 months (range, 16 to 72). At the time of diagnosis, most patients had been treated with single first-line chemotherapy regimens, although some patients had been treated with multiple chemotherapy regimens. Thirteen patients underwent autologous stem cell transplantation (ASCT).
Prognostic factors
The known prognostic factors for patients with MM were included in the analysis. These consist of age (≥65 years), albumin (<3.5 g/L), B2MG (≥3.5 µg/mL), BMPC (≥40%), creatinine (≥2.0 mg/dL), CRP (≥2.0 mg/dL), hemoglobin (<8.0 g/dL), International staging system (ISS) stage (I to III), Eastern Cooperative Oncology Group performance status (ECOG ≥2), conventional cytogenetics (normal or abnormal karyotype), and ASCT. The results of fluorescence in situ hybridization (FISH) were not included, because FISH was performed on only 23% of the patients.
A colorimetric enzyme-linked immunosorbent assay was used to quantify the serum ferritin level at diagnosis. The normal range for serum ferritin in our laboratory is 18-300 ng/mL. Therefore, the cut-off level of serum ferritin was set at 300 ng/mL.
Statistical analysis
The overall survival (OS) was analyzed using the Kaplan-Meier method and measured from the date of diagnosis to the date of death or last follow-up. The Mann-Whitney U test was used to compare variables between the patients with and without elevated ferritin levels. The outcomes were estimated using the Kaplan-Meier method and compared between patients with and without elevated ferritin and according to the ISS stage. Cox proportional hazards analysis was used to identify univariate and multivariate prognostic factors for OS. The results of the Cox proportional hazards analyses are summarized as the hazard ratio (HR), or relative risk (RR), and 95% confidence interval (CI) for the HR. A p value <0.05 was considered to be statistically significant. Analyses were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
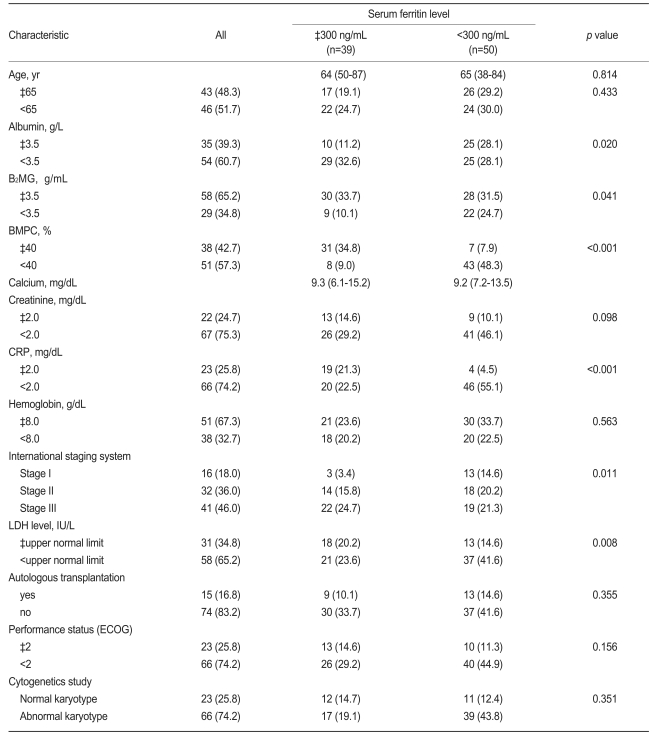
The distributions of the patients' baseline characteristics are presented in Table 1, according to the serum ferritin level at the time of diagnosis.
The initial treatment regimens were as follows: 47 patient received melphalan-prednisone; 21 patients, a thalidomide-containing regimen (thalidomide-dexamethasone or thalidomide-cyclophosphamide-dexamethasone); 13 patients, vincristine-adriamycin-dexamethasone and then ASCT; and eight patients, a bortezomib-containing regimen (bortezomib-dexamethasone).
The baseline characteristics were analyzed according to the serum ferritin. Thirty-nine MM patients had elevated ferritin, and 50 patients had normal ferritin levels. The patient group with an elevated serum level was associated with a lower albumin level, and B2MG, BMPC, CRP, and LDH levels above the upper limits of normal, compared with the patients with a normal ferritin level. The calcium, creatinine, and hemoglobin levels did not differ between the two patient groups (Table 1).
Outcome and prognostic factors
Sixty-three patients (70.8%) had died by the date of the final analysis. The median OS times according to the ISS stage were 39, 17, and 8 months for stages I, II, and III, respectively.
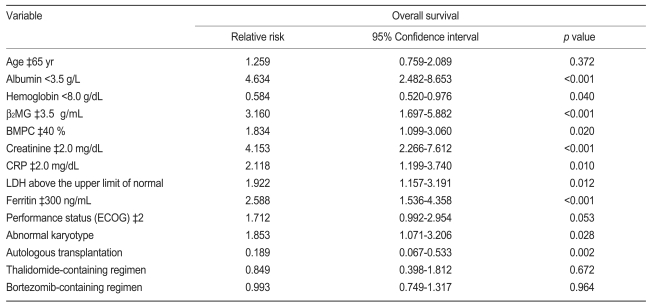
Based on univariate analysis using a Cox proportional model, the OS did not differ between the two age groups in this study (<65 and ≥65 years). However, higher albumin (p<0.001) and hemoglobin (p=0.040) levels, normal B2MG (p<0.001), BMPC (p=0.020), creatinine (p<0.001), CRP (p=0.010), and LDH (p=0.012) levels, an abnormal karyotype (p<0.001), and ASCT (p=0.002) were significantly associated with longer survival (Table 2). Furthermore, OS differed significantly according to the ISS stage (p<0.001, Fig. 1).
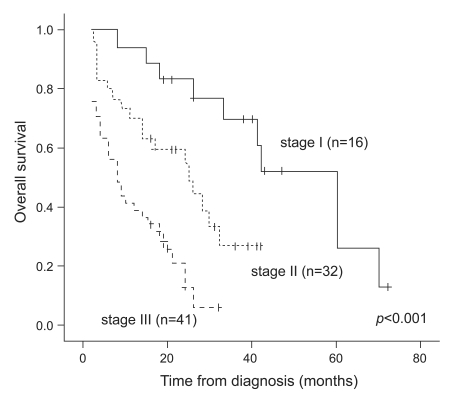
Overall survival according to the International staging system. The difference was statistically significant (p<0.001).
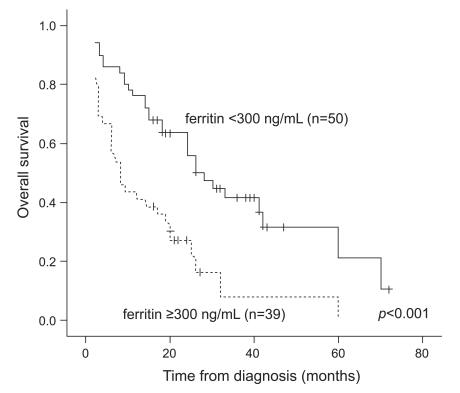
Interestingly, the group with elevated serum ferritin had poorer survival (p<0.001, Table 2 and Fig. 2). Twenty (40%) of the 50 patients in the normal ferritin group were alive at the end of the study, versus only seven (17.9%) of the 39 patients in the elevated ferritin group.
Prognostic value of serum ferritin and other factors
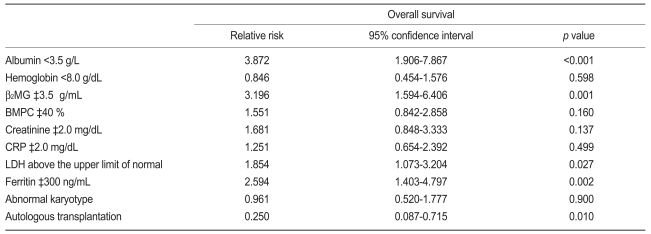
To further evaluate the prognostic value of the serum ferritin level in patients newly diagnosed with MM, Cox analysis was performed for ferritin and other factors. Based on multivariate analysis, a lower albumin (p<0.001; RR, 3.872; 95% CI, 1.906 to 7.867), higher B2MG (p=0.001; RR, 3.196; 95% CI, 1.594 to 6.406), elevated LDH (p=0.027; RR, 1.854; 95% CI, 1.073 to 3.204), and elevated ferritin (p=0.002; RR, 2.594; 95% CI, 1.403 to 4.797) were independent adverse prognostic factors associated with increased mortality. Furthermore, the patients who underwent ASCT had lower mortality (p=0.010; RR, 0.250; 95% CI, 0.087 to 0.715; Table 3).
DISCUSSION
The serum ferritin level reflects acute phase reactions and is usually associated with iron storage. Iron overload increases the susceptibility to organ damage and the risk for infection [8,9]. Recent studies have shown that serum ferritin is a surrogate of iron overload and is an important predictor of survival in transplantation patients [5,6]. However, one study showed that patients with hematologic malignancies have significantly higher serum ferritin levels [7]. Another study showed that the intensity of the 99mTc-MIBI scan correlates with the serum ferritin level as a marker of disease activity [10]. A third study found that elevated serum sIL-6R levels were related to the growth of myeloma cells and that the concentration was an indicator of disease activity; the sIL-6R level was correlated with the serum B2MG, CRP, ferritin, and LDH concentrations as a marker of disease activity [11]. However, no study has examined whether the serum ferritin level at diagnosis is associated with the outcome in patients newly diagnosed with MM.
The correlation between elevated ferritin levels and OS is unclear. A recent study reported that the urinary hepcidin level was significantly higher in MM patients than in normal controls and that it was correlated with the serum ferritin level [12]. The authors suggested that the growth of myeloma was associated with hepcidin-inducing cytokines and other mechanisms. The serum hepcidin concentration was also elevated in patients with MM in another study [13].
Hepcidin synthesis is pathologically increased by inflammation [14]. Hepcidin and serum ferritin respond similarly to inflammation and changes in iron stores, and this is reflected by the strong correlation between hepcidin and ferritin. Therefore, the serum ferritin level may reflect disease activity in MM.
In this study, the patients in the elevated ferritin level group had more adverse prognostic factors at baseline and a poorer clinical outcome compared with patients in the normal ferritin group. Furthermore, the serum ferritin levels at the time of diagnosis were correlated with survival in newly diagnosed MM patients. When the cut-off level of serum ferritin was set at 300 ng/mL, the median OS differed between the elevated and normal ferritin groups. Based on univariate analysis, the elevated serum ferritin group had a shorter survival time than the normal serum ferritin group. In addition, lower albumin, lower hemoglobin, higher B2MG, higher BMPC, higher creatinine, higher CRP, elevated LDH, undergoing conventional chemotherapy only, and an abnormal karyotype were adverse prognostic factors. Multivariate analysis showed that lower albumin, higher B2MG, elevated LDH, elevated ferritin, and no ASCT were independent prognostic factors for shorter survival in newly diagnosed MM patients.
This study has some limitations. First, a small number of MM patients was included and evaluated retrospectively. A prospective study with more patients should be performed. Second, the prognosis according to FISH was not determined and should be studied. Third, the outcomes according to treatment regimen were not measured. This was difficult to assess because of the many regimens administered.
In conclusion, the serum ferritin level may be associated with OS in patients with newly diagnosed MM. Further investigation is warranted to determine whether serum ferritin is an independent prognostic marker and a measure of disease activity.
Acknowledgements
This work was supported for two years by Pusan National University Research Grant.