Acceptance and Understanding of the Informed Consent Procedure Prior to Gastrointestinal Endoscopy by Patients: A Single-Center Experience in Korea
Article information
Abstract
Background/Aims
Only a few reports have examined informed consent for gastrointestinal endoscopy in Korea. The aim of this study was to evaluate the appropriateness of the informed consent procedure in Korea.
Methods
A total of 209 patients who underwent endoscopy were asked to answer a self-administered structured questionnaire on the informed consent procedure for gastrointestinal endoscopy.
Results
One hundred thirteen patients completed questionnaires and were enrolled. In the survey, 91.2% answered that they understood the procedure, and the degree of understanding decreased with age; 85.8% were informed of the risks of the procedure, and the proportion was higher for inpatients and for those receiving therapeutic endoscopy or endoscopic retrograde cholangiopancreatography; 60.2% were informed of alternative methods, and the proportion was higher in older patients; 76.1% had the opportunity to ask questions during the informed consent procedure, and the proportion was higher in inpatients. The understanding of the risks of the endoscopic procedure was better in the younger and more highly educated groups. About 80% had sedation before endoscopy, and only 56% were informed of the risks of sedation during endoscopy.
Conclusions
The current informed consent process may be reasonably acceptable and understandable to the patients. However, the understanding of the risks of endoscopy was insufficient especially in the cases of older, poorly educated patients and outpatients. The information about alternatives, the opportunity to ask for additional information, and the information about the risks of sedation during endoscopy were also insufficient in the current consent process.
INTRODUCTION
The doctrine of informed medical consent embodies the legal recognition of the right of an individual to make health care decisions affecting his or her well being [1]. Initially, the consent process had a provider-based standard with regard to the information that must be presented. However, the concept has been moving toward a patient-based standard for information that should be disclosed to obtain a truly informed consent [2]. Negligent failure to inform has been considered a very important reason for liability claims against doctors.
The elements of adequate disclosure should include 1) the reason for the procedure; 2) the nature (description) of the proposed procedure, i.e., what will happen before, during, and after the procedure; 3) the diagnostic benefits, including therapeutic options; 4) the possible risks, complications, and mortality rates of the procedure; 5) reasonable alternatives to the proposed procedure and their advantages and disadvantages; and 6) a clear explanation of the patient's right to withdraw consent at any time prior to the procedure [3].
Gastrointestinal endoscopies, even diagnostic endoscopies, can cause procedure-associated complications, so obtaining informed consent from patients prior to the procedure is regarded as an essential step for gastrointestinal endoscopy. However, some controversies remain about the appropriateness of the current informed consent procedure prior to gastrointestinal endoscopy and its acceptance and understanding by patients. A previous study showed that the current consent process was inadequate for educating patients about the possible serious risks involved and about other therapeutic options [4]. Additionally, a survey performed in the UK showed that the consent procedures appeared to fall short of the ideal, particularly with regard to information about procedural risks and allowing patients sufficient time to make informed decisions [5]. However, another study reported that nearly all patients understood the nature of and the reasons for the test before consenting [6].
The aim of this study was to evaluate the appropriateness of the informed consent procedure prior to gastrointestinal endoscopy and its acceptance and understanding by patients in Korea.
METHODS
Between November 2006 and January 2007, 209 patients who had undergone endoscopy (diagnostic colonoscopies, therapeutic gastroscopies and colonoscopies such as endoscopic mucosal resections, endoscopic submucosal dissections or electrocauterization, and endoscopic retrograde cholangiopancreatographies [ERCP]) at Samsung Medical Center were asked to complete a self-administered, structured questionnaire on the informed consent procedure for gastrointestinal endoscopy. Patients undergoing diagnostic gastroscopies were not included in this study, as we usually obtain only verbal informed consent before this procedure. A total of 113 patients agreed to complete the questionnaire and were enrolled in this study. This study received approval from the institutional review board of the Samsung Medical Center, and patients signed a research consent form before participating in the study.
The questionnaire consisted of questions about the patient's demographics and the following questions.
What kind of procedure did you undergo?
How many times have you undergone endoscopic procedures during the last 5 years?
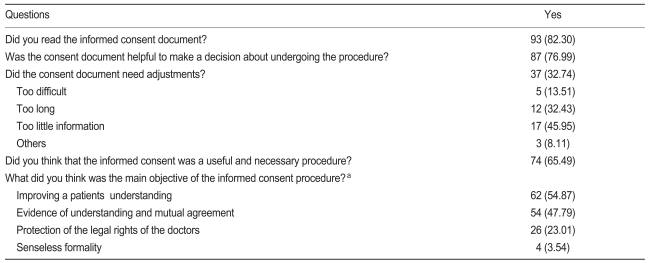
Did you read the informed consent document prior to the procedure?
Who obtained the informed consent from you? doctor? nurse? other? (multiple-choice question)
Was the consent document helpful in making a decision about undergoing the procedure?
Did the consent document need any adjustments? What was the main problem with the consent document?
Did you think that informed consent was a useful and necessary procedure? What did you think was the main objective of the informed consent procedure? Was it a process for improving a patients' understanding of a specific procedure, or was it just for releasing doctors from their responsibility?
Do you know the reason for the proposed endoscopy?
Do you understand the details of the proposed endoscopy?
Were you informed of the associated risks of the proposed endoscopy? What is(are) the possible complication(s) of the endoscopic procedure? (multiple-choice question)
Were you informed of the alternative methods other than the proposed endoscopy?
Were you given an opportunity to ask questions during the informed consent procedure?
If you were expected to receive sedation during endoscopy, were you given general information about sedation? Were you informed of the associated risks of sedation during endoscopy? What is(are) the possible complication(s) of the use of sedation in endoscopy? (multiple-choice question)
Statistical analysis was performed using the chi-square, Fisher's exact, or the Wilcoxon tests to compare the differences between the two groups and by the Kruskal-Wallis test or analysis of variance to compare the differences among the three groups. Additionally, the Spearman correlation coefficient was used to determine associations between continuous variables. The factors that had a potential to correlate with patients' understanding of the specific risks for the endoscopic procedures were subjected to multivariate analysis with the logistic model. All p values were two-tailed, and p values less than 0.05 were considered statistically significant.
RESULTS
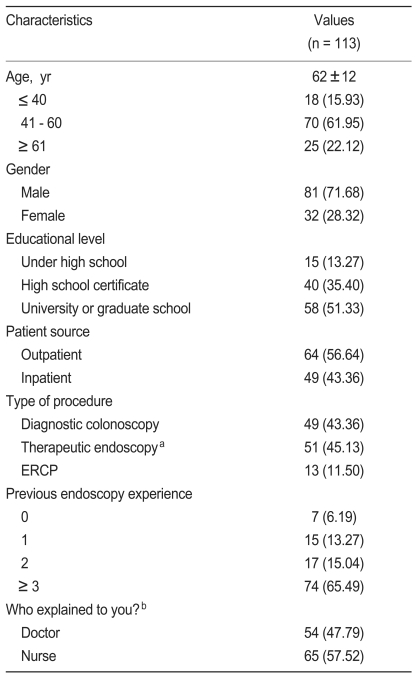
Table 1 summarizes the demographic details of the 113 patients. Of the 64 outpatients, 46 patients (71.9%) received a diagnostic colonoscopy, 18 (28.1%) a therapeutic endoscopy, and no one received ERCP. Of the 49 inpatients, three patients (6.1%) received a diagnostic colonoscopy, 33 (67.3%) a therapeutic endoscopy, and 13 (26.5%) an ERCP.
Patient perceptions of the informed consent procedure are shown in Table 2. The perception of the informed consent process was positive for 85.0% of all patients surveyed.
When patients were asked about whether they knew the reason for the proposed procedure, most (99.1%) answered that they knew the reason.
Regarding the details of the proposed endoscopy, i.e., what will happen before, during, and after the procedure, 103 patients (91.2%) answered that they understood the procedure well. As to being informed of the risk of complications associated with endoscopy, 97 patients (85.8%) answered that they were informed of the possible risk of complications. Table 3 shows the factors affecting the patients' understanding of the process and possible complications of the endoscopic procedure.
Of the 97 patients who were informed of the risks of endoscopy, 97.9% (n = 95) answered that they knew bleeding was a procedural risk, 74.2% (n = 72) were aware of possible perforations, 46.4% (n = 45) were aware of possible infections, 42.3% (n = 41) were aware of possible respiratory difficulties, 27.8% (n = 27) were aware of possible blood pressure decreases, and 10.3% (n = 10) were aware of the possibility of death.
Factors related to the patients' understanding of the specific risks of the endoscopic procedure are shown in Table 4.

Univariate analysis of the factors related to the patients' understanding of the risks of endoscopic procedures
The four factors that had a potential to correlate with the patients' understanding of the risks of endoscopic procedures, namely, age, educational level, patient source, and type of procedure, were subjected to multivariate analysis (Table 5). The middle-aged group (41 to 60 years) was more knowledgeable about the perforation risk (odds ratio [OR], 5.06; p = 0.008), and the younger-aged (< 40 years) and middle-aged groups were more knowledgeable about the respiratory problem as a risk of the endoscopic procedure than was the older-aged group (> 60 years) ([OR, 5.13; p = 0.036], [OR, 3.29; p = 0.043], respectively). The highly educated group (university or graduate school) was more knowledgeable about bleeding (OR, 9.38; p = 0.044) and perforation (OR, 10.77; p = 0.003) than was the least-educated group (less than high school). Inpatients were more knowledgeable about infection than were outpatients (OR, 3.74; p = 0.031).
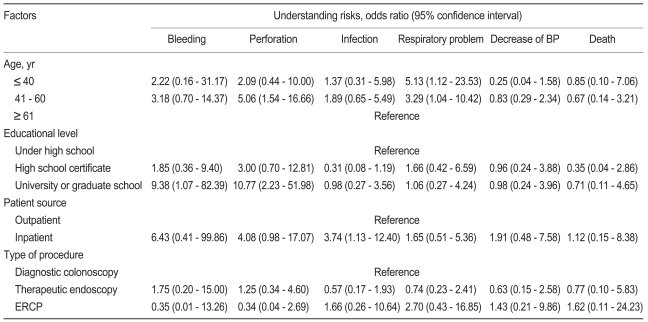
Multivariate analysis of the factors related to the patients' understanding of the risks of endoscopic procedures
Sixty-eight patients (60.2%) answered that they were informed of alternatives to the procedure. The proportion of older patients who were informed of alternatives was higher than that of younger patients (p = 0.045).
Eighty-six patients (76.1%) answered that they had the opportunity to ask questions. The proportion of inpatients who had the opportunity to ask questions was higher than that of outpatients (p = 0.036).
A total of 80.5% (n = 91) of the patients were sedated with midazolam during the endoscopy. Seven patients (7.7%) were not given general information about sedation, and all of them had undergone a more than three previous endoscopies. Fifty-one patients (56%) were informed of the risks of sedation during endoscopy. Younger patients knew the risks more often than older patients did (r = -0.1899, p = 0.044). When we asked the patients for the specific risks of sedation with endoscopy to test whether they were informed in detail, 48.4% of the patients answered that they knew of respiratory difficulties as a complication associated with sedation, 29.7% mentioned hypersensitivity, 19.8% tachycardia, 12.1% respiratory arrest, 11.0% hypoxia, 7.7% falling down, and 4.4% cardiac arrest.
DISCUSSION
Concerns about medical/legal issues and the demands for established informed consent guidelines are increasing. Several studies have examined the practice of informed consent for gastrointestinal endoscopy in Western countries [7-12].
However, only a few studies have been reported in Eastern countries. The present study attempted to evaluate the quality of the current informed consent process for gastrointestinal endoscopy in Korea.
It is recommended that the doctor suggesting the procedure should describe the essential elements of the procedure. The endoscopist would be best advised to personally obtain a patients' informed consent [13,14]. Our study showed that informed consent was obtained by the doctor (whether a gastroenterologist or physician in a hospital ward) or the endoscopic nurse, but only 47.8% of the patients surveyed were informed by doctors. A study performed in China showed similar results. Other patients were informed by doctors and nursing staff in clinics or in hospital wards [15]. Another survey in Europe showed that, in 23% of countries, the endoscopist was responsible for providing information and obtaining informed consent, and in 62% of the countries it was the endoscopy assistant or nurse [11]. Recently, the Department of Health in the UK advised that informed consent may be obtained by someone who is not an endoscopist but who has received appropriate training to obtain informed consent for a specific procedure [11]. Patients whose consent was obtained by doctors were more informed of the procedural risks, but no significant differences were found in patients' recognition of specific risks. Furthermore, no significant differences were observed in other elements of the process such as information on procedural details and alternatives to the procedure and opportunities to ask questions. These results showed no differences in patients' understanding of the proposed procedure and essential elements of the informed consent process whether consent was obtained by a doctor or nurse. Thus, it might be acceptable that informed consent be obtained by endoscopic nurses who are specially trained to counsel patients on the procedure.
About one-third of patients thought that informed consent would not be necessary, suggesting many deficiencies in the current process for obtaining valid informed consent. Unfortunately, rather than fostering communication as intended, many clinicians view obtaining written, informed consent as an obstacle: they must get a form signed so that they can proceed with their work [16]. Clinicians must be mindful of the fact that informed consent is a process of disclosure and deliberation, not merely a matter of signing a form [14]. The aim is not simply consent but, "informed consent," and this requires that the patient be given reasonably sufficient information about the diagnosis, treatment, and chances of success for the proposed treatment as well as the risks involved in undergoing or foregoing the procedure [17].
Providing patients with printed and audio-visual materials can be helpful for patient decision making [18]. The use of videotapes in the informed consent process for colonoscopy is effective for helping patients to understand the risks and benefits of the procedure [19]. Moreover, graphs, schematics, and other video wizardry would more fully demonstrate the procedure [20].
The majority of patients in our study (82.3%) read the informed consent document, and 63.7% of these patients thought the document was understandable. No relationship was observed relationship between the degree of understanding and educational level. These findings are different from the results of previous studies [9]. The overall distribution of educational level differed among these studies, and the proportion of patients with basic education (13.3%) in our study population was relatively small, which may account for the difference. One report has indicated that adequate cognitive function does not predict a high level of understanding of the informed consent process, whereas cognitive impairment precludes it [21]. One-third of our patients thought the consent document needed some adjustments. For better informed consent, it would be necessary to develop a revised document written more simply, with easier wording and added information that patients want to know. Most patients appear to make a judgment about information requirements on the basis of the incidence and consequences of the complications [22]. A majority of the patients wished to know of risks greater than 1 in 1000 [10], but some wanted information about all complications, irrespective of risk and severity [22].
The risk of complications for the endoscopic procedure was explained more frequently to younger inpatients and to patients receiving therapeutic endoscopy or ERCP. The multivariate analysis revealed that the understanding of the risks was better in younger, more highly educated patients and in inpatients than outpatients. Most patients were informed about bleeding or perforation as risks of endoscopy, but infection, respiratory problems, decreases in blood pressure, and death were not commonly explained as possible risks of endoscopy. The risks of sedation during endoscopy were not sufficiently explained to the patients.
This is the first study to evaluate the quality of the current informed consent process for gastrointestinal endoscopy in Korea. However, this study had important limitations, mostly stemming from its being a single center study and its small sample size.
In conclusion, we found that the informed consent process was reasonably acceptable and understandable to patients who underwent a gastrointestinal endoscopy. However, the understanding of the risks of endoscopy was insufficient, especially among older, less educated inpatients and among outpatients. The information concerning alternatives, opportunities to ask for additional information, and the information about the risks of sedation during endoscopy were also insufficient in the current consent process. An educational program to explain the informed consent procedure and its importance may help improve the informed consent process in Korea.
Notes
No potential conflict of interest relevant to this article was reported.