Combination of Uric Acid and NT-ProBNP: A More Useful Prognostic Marker for Short-Term Clinical Outcomes in Patients with Acute Heart Failure
Article information
Abstract
Background/Aims
In patients with heart failure (HF), N-terminal prohormone brain natriuretic peptide (NT-ProBNP) is a standard prognostic indicator. In addition, uric acid (UA) was recently established as a prognostic marker for poor outcome in chronic HF. The aim of this study was to determine the combined role of UA and NT-ProBNP as prognostic markers for short-term outcomes of acute heart failure (AHF).
Methods
The levels of UA and NT-ProBNP were determined in 193 patients (age, 69 ± 13 years; 76 males) admitted with AHF. Patients were followed for 3 months and evaluated for cardiovascular events, defined as cardiac death and/or readmission for HF.
Results
Of the 193 patients, 23 (11.9%) died and 20 (10.4%) were readmitted for HF during the 3-month follow-up period. Based on univariate analysis, possible predictors of short-term cardiovascular events were high levels of UA and NT-ProBNP, low creatinine clearance, no angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and old age. Multivariate Cox hazard analysis showed that UA levels were independently associated with increased incidence of cardiovascular events (hazard ratio, 1.115; 95% confidence interval, 1.006 to 1.235; p = 0.037). Kaplan-Meier survival analysis revealed that patients with UA levels > 8.0 mg/dL and NT-ProBNP levels > 4,210 pg/mL were at highest risk for cardiac events (p = 0.01).
Conclusions
The combination of UA and NT-ProBNP levels appears to be more useful than either marker alone as an independent predictor for short-term outcomes in patients with AHF.
INTRODUCTION
Heart failure (HF) is the one of the most common cardiovascular disorders and is associated with high morbidity and mortality. Although numerous parameters indicate the prognosis of heart failure, most are costly to evaluate and are assessed only in research. There is a need for prognostic parameters that can be measured simply and inexpensively in any setting.
Previous studies have demonstrated that N-terminal prohormone brain natriuretic peptide (NT-ProBNP) levels have diagnostic and prognostic value in patients with HF. The NT-ProBNP level was a useful marker in the diagnosis of HF for complaints of acute dyspnea [1,2]. Other studies have suggested that in patients with HF, the NT-ProBNP level is an important predictor for clinical outcomes such as hospital readmission and mortality [3,4].
Numerous parameters predict the prognosis of HF. Recently, a number of studies have reported an association between the uric acid (UA) level and cardiovascular risk factors or adverse cardiovascular outcomes [5-11]. Elevated UA levels were also associated with HF. A high serum UA level was an independent prognostic marker in patients with moderate to severe chronic heart failure (CHF) [12]. However, the prognostic impact of serum UA levels in patients with acute heart failure (AHF) is not well defined. Moreover, the impact of combined UA and NT-ProBNP levels as a prognostic marker in AHF is poorly understood.
The purpose of this study was to assess the prognostic value of the combination of UA and NT-ProBNP levels as an indicator of short-term outcomes in patients with AHF.
METHODS
Study population
A total of 193 consecutive patients who were admitted to the emergency department because of AHF were studied retrospectively. AHF was defined as a rapid onset of signs and symptoms secondary to abnormal cardiac function. AHF could present as acute de novo (new onset HF in a patient without previously known cardiac dysfunction) or acute decompensation of CHF [13].
Laboratory measurements
Blood samples were obtained intravenously immediately after admission. The serum UA level was measured by a uricase-peroxidase method (ADVIA® 1650 Chemistry System, Siemens Medical Solutions Diagnostics, Tarrytown, NY, USA), and the plasma NT-proBNP level was measured by immunoassay (Dimension® RxL Max® Integrated Chemistry System, Siemens Healthcare Diagnostics Inc, Deerfield, IL, USA). Other biochemical parameters were measured according to standard techniques. The rate of creatinine clearance (CrCl, mL/min) was determined using the Cockcroft-Gault equation [14].
Study endpoint
The endpoint of this study was cardiovascular death and/or readmission for heart failure within 3 months after the initial admission.
Statistical analysis
Continuous variables are expressed as mean ± standard deviations. Independent groups were compared using the unpaired Student's t test. Categorical data were compared with the chi-squared test, and Fisher's exact test was performed when relevant. The NT-ProBNP values were log-transformed to reduce the effect of extreme values, because the relationship between the NT-proBNP level and the endpoint was not linear. Receiver operating characteristic (ROC) curves were used to determine the cut-off values for biochemical parameters. The optimal values of UA and NT-ProBNP for predicting cardiac events were defined as the concentrations with the largest sensitivity plus specificity for the curves. Survival was analyzed with Kaplan-Meier cumulative survival curves.
Differences in the survival rate were evaluated using the log-rank test. Independent prognostic indicators for clinical outcomes were evaluated by univariate and multivariate Cox proportional hazard analysis. The results are expressed as the hazard ratio (HR) and 95% confidence interval (CI). Variables included in the multivariate analysis were known risk factors and variables with p < 0.10 in the univariate analysis. The incremental prognostic values of the UA and NT-ProBNP levels compared with conventional risk factors were assessed by global chi-square values calculated after adding in several independent predictors identified by multivariate analysis, based on increases in the overall likelihood ratio. The incremental factors added to the model at each step were considered significant when the difference in log-likelihood associated with each model corresponded to p < 0.05. Statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was defined at p < 0.05.
RESULTS
Baseline characteristics
This study included 193 consecutive patients (age, 69 ± 13 years; 76 males) who presented to the emergency department of a tertiary care hospital because of AHF. During a 3-month follow-up, 23 patients (11.9%) died of cardiovascular events and 20 patients (10.4%) were readmitted for HF. The causes of cardiovascular deaths were cardiogenic shock, pulmonary edema due to worsened heart failure, and sudden death probably attributable to ventricular arrhythmia.
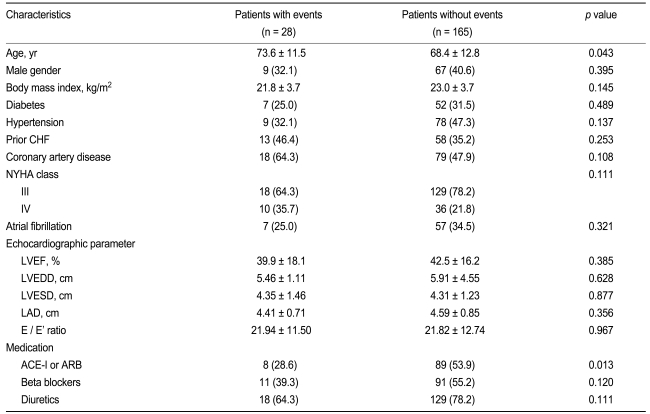
The baseline characteristics of the study subjects are given in Table 1. Patients with cardiovascular events (n = 28) were older than those without events (n = 165), and patients who had received angiotenin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) were less likely to have cardiovascular events. However, the rates of diabetes and hypertension were similar between the groups with and without cardiovascular events, and there were no differences in echocardiographic parameters between the two groups.
Biochemical parameters
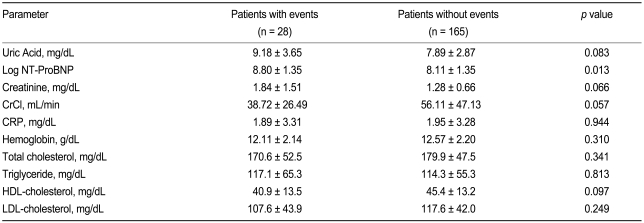
Table 2 presents a comparison of biochemical parameters between the groups with or without cardiovascular events. Compared with patients without events, patients with cardiovascular events showed significantly higher levels of NT-ProBNP and UA, and a greater deterioration of renal function parameters. However, no other biochemical parameters differed significantly between the groups.
Predictors for cardiovascular events
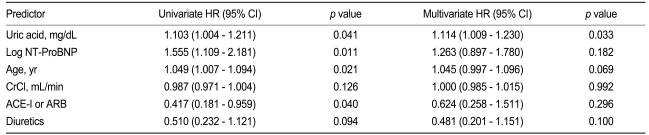
The predictors of cardiovascular events based on univariate and multivariate analyses are shown in Table 3. The variables with significant predictive value in the univariate Cox hazard analysis as well as conventional risk factors were used for the multivariate analysis. The univariate analysis revealed that patients with a high UA level, a high NT-ProBNP level, and old age are at higher risk for short-term cardiovascular events. Patients who did not receive ACE inhibitors or ARBs also had a higher risk of cardiovascular events. In the multivariate analysis, a high UA level was the only significant independent prognostic indicator for cardiovascular events (HR, 1.114; 95% CI, 1.009 to 1.230; p = 0.033).
Fig. 1 shows the ROC curves for the UA and NT-ProBNP levels and the prediction of events. The area under the ROC curve was similar between UA and NT-ProBNP (0.623; 95% CI, 0.494 to 0.751, and 0.642; 95% CI, 0.528 to 0.757, respectively). For UA, the optimal level was 8.0 mg/dL, with a sensitivity of 67.9% and specificity of 60.4%. For NT-ProBNP, the optimal level was 4210pg/mL, with a sensitivity of 71.4% and specificity of 50.3%.
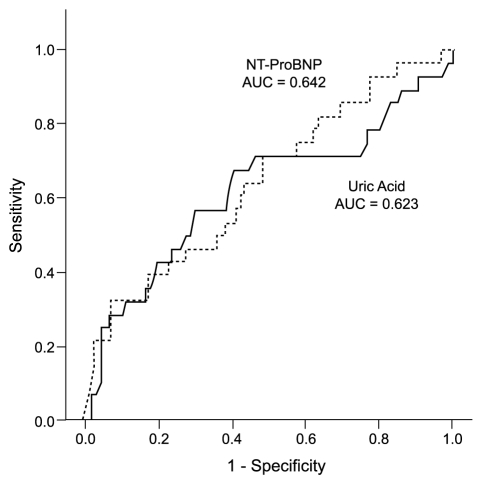
Receiver-operating characteristic curves for uric acid and N-terminal prohormone brain natriuretic peptide (NT-proBNP) levels vs. cardiovascular events. AUC, area under the curve.
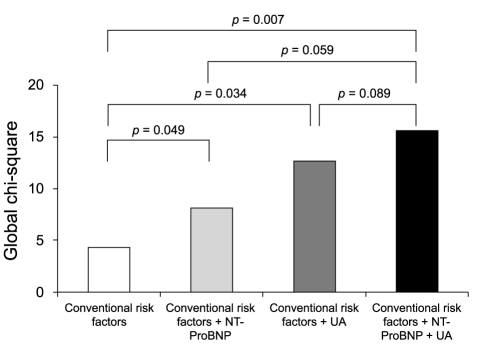
The Kaplan-Meier survival curve for combined cardiovascular events showed that patients with a UA level > 8.0 mg/dL and NT-ProBNP level > 4,210 pg/mL were at higher risk for cardiovascular events, compared with patients with UA ≤ 8.0 mg/dL and NT-ProBNP ≤ 4,210 pg/mL (HR, 5.840; 95% CI, 1.677 to 20.333; p = 0.006) (Fig. 2). The global chi-square analysis showed an incremental, although not statistically significant, improvement of 4 in the prognostic value with the addition of the NT-ProBNP level and of 8 with the addition of the UA level, compared with the prognostic value of conventional risk factors such as age and creatinine clearance. The addition of the combined NT-ProBNP and UA levels gave a greater incremental increase in the prognostic value (Fig. 3).

Kaplan-Meier survival curves stratified by the combination of uric acid (UA) and N-terminal prohormone brain natriuretic peptide (NT-ProBNP).
DISCUSSION
This study demonstrated that UA was an important prognostic factor for short-term clinical outcome in patients with AHF. Moreover, the combination of the UA and NT-ProBNP levels had additional value for predicting the prognosis in patients with AHF.
UA is the final product of purine metabolism [15], and hypoxic states cause an elevation of the serum UA level [16]. The activation of the xanthine oxidase (XO) system by tissue hypoxia increases UA production and causes hyperuricemia. Thus, hyperuricemia is a marker for impaired oxidative metabolism, and UA levels reflect the degree of XO activation [16]. Cell death, tissue hypoxia, and impaired metabolism in HF increase XO activity, leading to an overproduction of UA. Increased UA in the circulation of HF patients may result from an increased generation of UA, a decreased excretion of UA ,or a combination of both; however, overproduction of UA appears to be the dominate factor accounting for elevated UA levels in CHF [17].
Numerous studies have demonstrated the prognostic importance of hyperuricemia in patients with CHF. Anker et al. [12] have reported that a high serum UA level is a strong, independent marker of poor prognosis in patients with moderate to severe CHF. Sakai et al. [18] have shown that UA is released from the heart in CHF patients, that the UA transcardiac gradient correlates with left ventricular ejection fraction, and that a high serum UA level is an important predictor of mortality, independent of factors such as glomerular filtration rate and BNP level, which are known predictors of poor prognosis in patients with mild to severe CHF. Other studies have suggested that the inhibition of XO may improve myocardial function [19] and ejection fraction [20] in patients with HF. Moreover, Struthers et al. [21] have demonstrated that long-term, high-dose allopurinol treatment, rather than long-term, low-dose allopurinol treatment, may be associated with decreased mortality in patients with CHF.
Recently, some studies have suggested that the UA level may be a prognostic marker in the setting of AHF. Pascual-Figal et al. [22] have reported the long-term clinical prognostic value of hyperuricemia in patients with AHF, showing that the UA level prior to discharge was associated with higher risk for death, heart failure readmission, and a combination of both events. The present study also suggested that serum UA has prognostic value in patients with AHF.
In several previous reports, the overproduction of UA by XO activation was the predominant underlying mechanism of hyperuricemia in CHF patients [19,23]. The pathophysiological role and mechanism of hyperuricemia may differ between AHF and CHF. However, there is no established mechanism to explain the cause of hyperuricemia in patients with AHF. In our opinion, cell death or apoptosis caused by ischemic insult or acute deterioration of renal function may be the dominant factor for hyperuricemia in AHF. More studies are warranted to determine the pathophysiological mechanism of hyperuricemia in patients with AHF.
Traditionally, BNP has been an important prognostic predictor in patients with HF [24,25]. The hypothesis of the present study was that the combination of the UA and NT-ProBNP levels also has a prognostic role in patients with AHF. In the present study, patients with a high UA or high NT-ProBNP level showed a poorer prognosis than patients with lower levels. Additionally, the combination of the UA and NT-ProBNP levels provided incremental prognostic value to that of conventional risk factors. Therefore, the combination of UA and NT-ProBNP levels appears to be a more powerful prognostic indicator for short-term clinical outcome in the setting of AHF, and risk stratification of patients according to the UA and NT-ProBNP levels is possible.
In this study, serum UA and NT-ProBNP levels obtained on admission were used for analysis. Biochemical markers obtained prior to discharge would have had limitations in predicting short-term clinical outcome, because they would not reflect the impact on clinical events such as in-hospital deaths. The purpose of this study was to assess the prognostic value of UA and NT-ProBNP levels in predicting short-term outcome; thus laboratory data obtained on admission were used for the analysis.
UA is excreted primarily by the kidney; therefore, a close relationship among the UA level, renal function, and diuretic treatment would be expected. However, previous studies have demonstrated that the prognostic value of UA is independent of these variables [22]. Multivariate analysis in the present study also showed that the prognostic value of UA is independent of renal function and diuretic treatment. The NT-ProBNP concentration is similarly affected by renal function. Nevertheless, the use of NT-ProBNP testing is valuable for the evaluation of patients with HF, irrespective of renal function [26].
In conclusion, although the UA level is an independent prognostic predictor for short-term clinical outcome in patients with AHF, the combination of UA and NT-ProBNP levels appears to be a more useful prognostic marker for short-term outcome in the setting of AHF.
Acknowledgements
This study was supported by a grant (RTI04-01-01) from the Regional Technology Innovation Program of the Ministry of Knowledge Economy (MKE) of South Korea.
Notes
No potential conflict of interest relevant to this article was reported.