Increased Expression of Cyclooxygenase-2 is Associated with the Progression to Cirrhosis
Article information
Abstract
Background/Aims
To investigate the degree of cyclooxygenase-2 (COX-2) protein expression in chronic hepatitis and cirrhosis.
Methods
COX-2 protein expression was evaluated in 43 cases of chronic hepatitis and 24 cases of cirrhosis using immunohistochemical techniques. The COX-2 immunohistochemical staining score was assessed using the scoring systems of Pazirandeh et al and Qiu et al. and each scoring system was based on a sum of the parameters of staining intensity and distribution.
Results
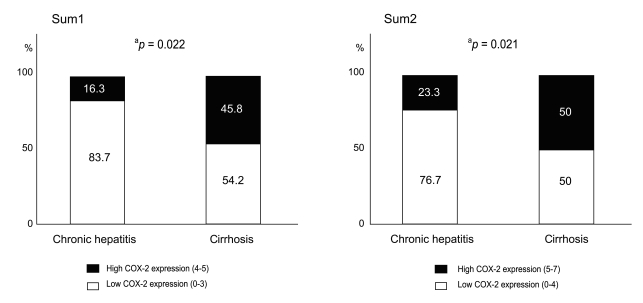
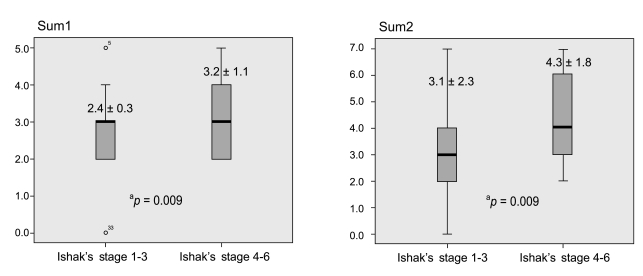
The mean COX-2 expression scores in chronic hepatitis and cirrhosis were 2.5 ± 1.3 vs. 3.3 ± 1.1 (p = 0.008), and 3.2 ± 2.0 vs. 4.5 ± 1.7 (p = 0.006), respectively, based on the Pazirandeh et al. and Qiu et al. scoring systems. The percentage samples of high COX-2 expression score (4 to 5) in chronic hepatitis and cirrhosis were 16.3% vs. 45.8% (p = 0.022), and 23.3% vs. 50% (p = 0.021), respectively, based on the two scoring systems. The mean COX-2 expression scores based on the severity of hepatic fibrosis scored using Ishak's modified staging system (fibrosis score 0 to 3 vs. 4 to 6) were 2.4 ± 1.3 vs. 3.2 ± 1.1 (p = 0.009), and 3.1 ± 2.0 vs. 4.3 ± 1.8 (p = 0.009), respectively, based on the two scoring systems.
Conclusions
COX-2 expression was significantly higher in liver cirrhosis group than in chronic hepatitis. COX-2 expression scores according to Ishak's staging was significantly higher in the advanced fibrosis group. COX-2 may play a role in the progression of hepatic fibrosis.
INTRODUCTION
Cyclooxygenase (COX)-1 and COX-2 are key enzymes that control the rate-limiting step in the conversion of arachidonic acid to prostaglandins. COX-1 is present constitutively in a number of different tissues, including the stomach, platelets, kidneys, and endothelial cells. It is responsible for regulating various physiological functions, including cytoprotection of the stomach, integrity of renal function, and platelet aggregation. Expression of the COX-2 enzyme is inducible through inflammatory and neoplastic processes involving a variety of factors such as mitogens, cytokines, growth factors, and tumor promoters [1-3]. COX-2 overexpression has been reported in various cancer models, including colon [2,3], esophageal [4], gastric [5], pancreatic [6], lung [7], and breast [8] cancers, as well as hepatocellular carcinoma (HCC) [9-12].
Hepatocarcinogenesis is a multistep process involving repeating cycles of hepatocyte inflammation, destruction, regeneration, and unregulated proliferation [13-17]. Although the exact pathogenesis of HCC is not fully understood, these changes are believed to be due, in part, to aberrant expression of various tumor suppressor genes, oncogenes, growth factors, and the COX-2 enzyme [17]. In addition to HCC, COX-2 is also upregulated in the liver under conditions of chronic viral hepatitis or cirrhosis [18,19]. Moreover, growing evidence indicates that COX-2 plays an important role in several signaling pathways in hepatic stellate cells (HSCs). Specifically, COX-2 is expressed in serum-starved HSCs in vitro, and its expression is further upregulated by cytokines [20,21]. HSC proliferation and migration stimulated by platelet-derived growth factor (PDGF) are associated with COX-2 induction and increased prostaglandin E2 production [21,22].
To clarify the role of COX-2 in the progression of liver fibrosis, the effects of treatment with COX-2 inhibitors have been investigated in animal models. The effects of COX-2 inhibitors on liver fibrosis, however, are controversial, with reports of both exacerbation [23-25] and amelioration [26-29] of fibrogenesis following COX-2 treatment. Direct comparisons of COX-2 expression in chronic hepatitis and cirrhosis have only rarely been reported, and a definitive role for COX-2 in fibrosis has not been fully elucidated. The present study was conducted to assess the degree of COX-2 expression in chronic hepatitis and liver cirrhosis and to identify differences in COX-2 expression associated with the severity of liver disease.
METHODS
Patients and liver specimens
Liver biopsies were selected from 91 cases between May 2003 and December 2006. The samples involved 50 cases of chronic hepatitis and 41 cases of liver cirrhosis. Following the exclusion of biopsies with inadequate material (n = 24), 67 cases were selected for staining: 43 cases of chronic hepatitis (hepatitis B/C, 21/22) and 24 cases of liver cirrhosis (hepatitis B/C/unknown, 14/6/4). Informed consent was obtained from each patient or from a family member.
COX-2 immunohistochemical staining
Biopsy samples were fixed in 10% neutral formalin and embedded in paraffin. Slide-mounted tissue sections were deparaffinized in xylene for more than 20 min and then hydrated sequentially in 100, 95, 90, and 80% ethanol solutions. After rinsing with water for 5 minutes, the sections were pretreated with EDTA buffer (pH 6.0) for 12 minutes using a microwave antigen-retrieval procedure. After rinsing, the endogenous peroxidase activity was blocked by treatment with 3% hydrogen peroxide for 20 minutes. A primary mouse monoclonal antibody against COX-2 (1 : 100; Cayman Chemical, Ann Arbor, MI, USA) was applied to the sections for 1 hour at room temperature. After rinsing with phosphate-buffered saline (PBS), the slides were incubated with a secondary antibody for 10 minutes at room temperature and then rinsed with PBS. The sections were incubated in tertiary anti-horseradish peroxidase (HRP) conjugate for 10 minutes, rinsed in PBS, and incubated with aminoethyl carbazole (AEC) for 10 minutes. After counterstaining with Meyer's hematoxylin, the slides were mounted with Crystal mount® (Biomeda, Foster City, CA, USA). The colon cancer tissue for the negative control slide was processed in the same way, except that samples were incubated in PBS instead of the primary antibody solution. Colon cancer tissue sections with known COX-2 staining status were used as positive or negative COX-2 staining controls (Fig. 1).
COX-2 immunohistochemical staining score
All of the stained biopsy samples were reviewed and interpreted by an expert hepatopathologist. Histological grading and staging were assessed using the Ishak's system [30]. The COX-2 immunohistochemical staining score was assessed using the scoring systems by Pazirandeh et al. [19] and by Qiu et al. [31], which are both based on a sum of two parameters: staining intensity and staining distribution. In the scoring system of Pazirandeh et al. [19], COX-2 staining intensity in tissue was scored using a scale of 0 to 3 (0 = no staining, 1 = weak staining, 2 = moderate staining, and 3 = strong staining), and the distribution of COX-2 staining was scored using a scale of 0 to 2 (0 = no staining, 1 = focal/patchy staining, 2 = diffuse staining). Each tissue was evaluated for the sum of these two parameters and assessed by the degree of COX-2 immunoreactivity using a qualitative score that ranged from 0 to 5. Scores between 0 and 3 were categorized as low COX-2 expression, and scores of 4 and 5 were categorized as high COX-2 expression.
The scoring system described by Qiu et al. [31] was used as follows. The intensity of staining for COX-2 in tissues was scored on a scale of 0 to 3 (0 = negative staining, 1 = weakly positive staining, 2 = moderately positive staining, and 3 = strongly positive staining). The percentage of positive cells in each specimen was estimated and scored on a scale of 0 to 4 (0 = negative, 1 = positive staining in 1 to 25% of cells counted, 2 = positive in 26 to 50%, 3 = positive in 51 to 75%, and 4 = positive in 76 to 100%). Each section was evaluated for the sum of these two parameters, with combined scores between 0 and 4 indicating low COX-2 expression and scores from 5 to 7 indicating high COX-2 expression. In this report, the scoring system of Pazirandeh et al. [19] is referred to as 'sum 1,' and the scoring system of Qiu et al. [31] as 'sum 2.'
Histological staging according to fibrosis
Ishak's system [30] was used to evaluate possible correlations between COX-2 expression and the stage of hepatic fibrosis. Scores for fibrosis stage ranged from 0 to 6. To identify the correlation of COX-2 expression with the stage of fibrosis, scores of COX-2 expression according to the degree of hepatic fibrosis were evaluated by categorization into a low-fibrosis (Ishak's score 1 to 3; n = 30) and a high-fibrosis stage (Ishak's score 4 to 6; n = 37).
Statistical analysis
The correlation coefficient was used to assess the relationship between the sum 1 and sum 2 scoring systems. The Mann-Whitney U test was used to compare patient characteristics and COX-2 expression scores between cases of chronic hepatitis and cirrhosis. Pearson's chi-square test was used to compare the percentage of high COX-2 expression between chronic hepatitis and cirrhosis. Student's t test was used to compare COX-2 expression scores between chronic hepatitis and cirrhosis and also to evaluate COX-2 expression scores according to the severity of hepatic fibrosis (Ishak's stage 1 to 3 vs. 4 to 6). Data are presented as the mean ± standard deviation (SD). Significance was accepted when the p value was less than 0.05.
RESULTS
Patient characteristics
Patient characteristics are presented in Table 1. The mean patient age was 46.5 ± 14.1 years. There were no significant differences between the chronic hepatitis and liver cirrhosis cohorts with regard to gender, bilirubin, or alpha-fetoprotein (AFP) expression. However, significant differences were observed for patient age, alanine transaminase (ALT), platelets, albumin expression, and prothrombin time (PT).
COX-2 immunoreactivity
Hepatocyte cytoplasmic staining for COX-2 was noted in cirrhotic and chronic hepatitis tissues as assessed by the two scoring systems. In Fig. 2, panels A to D present the intensity score range from 0 to 3, and panels E and F show the distribution scores from 0 to 2 based on the scoring system of Pazirandeh et al. [19]. The latter can be converted to 1 (1 to 25%) and 3 (51 to 75%), respectively, in the system of Qiu et al. [31]. When COX-2 expression in tissue samples from patients with chronic hepatitis (n = 43) and liver cirrhosis (n = 24) were assessed using the two scoring systems, the correlation coefficient between these two scoring systems was r = 0.965 (p < 0.001).
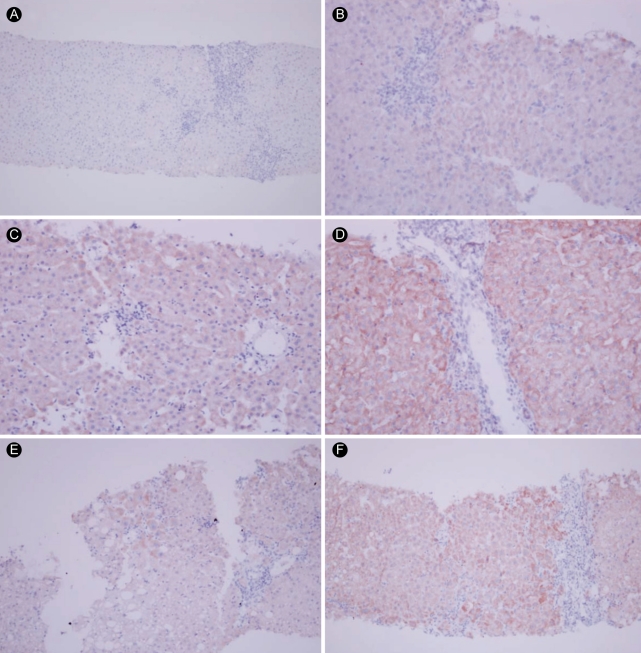
Immunohistochemical findings of cyclooxygenase-2 expression. Representative images depicting positive staining as scored using two different scoring systems. Intensity scores of (A) 0 (negative, × 100); (B) 1 (weak, × 200); (C) 2 (moderate, × 200); and (D) 3 (strong, × 200). (E) A distribution score of 1 (focal) using Pazirandeh et al. [19] and 1 (1 to 25%) using Qiu et al. [31] (× 100). (F) A distribution score of 2 (diffuse) using Pazirandeh et al. and 3 (51 to 75%) using Qiu et al. (× 100).
Comparison of COX-2 expression in chronic hepatitis and cirrhosis
Mean COX-2 expression scores for chronic hepatitis and liver cirrhosis were 2.5 ± 1.3 and 3.3 ± 1.1, respectively, for sum 1 (p = 0.008), and 3.2 ± 2.0 and 4.5 ± 1.7, respectively, for sum 2 (p = 0.006, Fig. 3). Using both methods, COX-2 staining scores were significantly different between the two groups. In terms of the percentage of tissue samples showing high COX-2 expression, a higher percentage of the cirrhosis group stained positively for COX-2 compared with the chronic hepatitis group. The percentage of chronic hepatitis and cirrhosis samples with high COX-2 expression (score 4 to 5) based on sum 1 were 16.3% and 45.8%, respectively (p = 0.022, Fig. 4), and 23.3% and 50.0%, respectively (p = 0.021, Fig. 4), based on sum 2 (score 5 to 7).

Cyclooxygenase-2 (COX-2) expression scores in chronic hepatitis and cirrhosis. COX-2 expression scores in chronic hepatitis and cirrhosis according to the scoring system of sum1 (A) and sum2 (B) as described in the Methods. aStudent's t test.
COX-2 expression and severity of hepatic fibrosis
COX-2 expression scores based on the degree of hepatic fibrosis according to Ishak's staging using the full range of scores (from 1 to 6) revealed no significant differences in sum 1 or sum 2 (r = 0.150, p > 0.05). However, when scores of COX-2 expression was assessed according to categorization based on low- vs. high-fibrosis stages (Ishak's stages 1 to 3 vs. stages 4 to 6), COX-2 expression scores for the stages 1 to 3 group and the stages 4 to 6 group were 2.4 ± 1.3 vs. 3.2 ± 1.1, respectively, for sum 1 (p = 0.009), and 3.1 ± 2.0 vs. 4.3 ± 1.8, respectively, for sum 2 (p = 0.009 Fig. 5).
DISCUSSION
The present study demonstrated that COX-2 expression is significantly higher in cirrhosis than in chronic hepatitis, as well as in advanced fibrosis among all tissues, suggesting that COX-2 is involved in the progression of cirrhosis and fibrosis. This is the first report to definitively show that COX-2 expression is higher in cirrhosis than in chronic hepatitis using two scoring systems to validate the results of the COX-2 expression scores. Sum 2 displayed a wider range of staining scores and was better able to identify faint differences in COX-2 expression in tissues compared with sum 1, but it showed the same results as those obtained using the sum 1 method.
COX-2 expression has been reported to be upregulated in chronic hepatitis, liver cirrhosis, and HCC [10,19] and is thought to be a mediator of liver damage and liver inflammation [32-34]. Pazirandeh et al. [19] reported a significant difference in the mean COX-2 staining scores between chronic hepatitis (2.2 ± 1.6) and cirrhosis (4.37 ± 1.15), as well as hepatocellular carcinoma (4.76 ± 0.54). Based on staining scores, 81.5% of cirrhotic tissues displayed a high COX-2 expression score (4 to 5) vs. only 17% of chronic hepatitis tissues [19]. In the present study, the percentage of high COX-2 expression scores (4 to 5) was 45.8% for liver cirrhosis and 16.3% for chronic hepatitis based on sum 1 (p = 0.022) and 50.0% for liver cirrhosis and 23.3% for chronic hepatitis according to sum 2 (p = 0.021). The reason for relatively low expression of COX-2 in cirrhotic tissues in our study compared with that of Pazirandeh et al. [19] is unclear. Patients with hepatitis C dominated in the group evaluated by Pazirandeh et al. (25 of 27 in the cirrhosis group), whereas patients with hepatitis B were the majority in our study (14 of 24 with hepatitis B vs. 7 of 24 with hepatitis C). Compared with the results reported by Pazirandeh et al., the percentage of samples showing high COX-2 expression in the hepatitis C group was lower in our study, and the percentage of high COX-2 expression in the hepatitis B group was higher. Specifically, the percentage of high COX-2 expression scores (4 to 5) was 50.0% (7/14) in the hepatitis B group and 14.3% (1/7) in the hepatitis C group. However, any possible influence of viral etiology on COX-2 expression cannot be compared directly because the disease activity was different, despite their being diseases of the same viral origin and same cirrhosis status. Finally, besides the differences in the etiology of hepatitis in each study, laboratory bias and racial and regional factors may also have influenced COX-2 expression.
COX-2 expression was also associated with the stage of fibrosis. When we categorized tissue samples as low-fibrosis (Ishak's score 1 to 3) and high-fibrosis stages (Ishak's score 4 to 6), a significant correlation was observed between COX-2 expression and the stage of fibrosis. Although the correlation between COX-2 expression and individual stages 1 to 6 showed no statistical significance, a significant increase in COX-2 expression was observed with high fibrosis when the fibrosis stages were divided into two groups (stages 1 to 3 vs. 4 to 6).
This study had the limitations associated with cross-sectional research; although the findings demonstrated high COX-2 expression in cirrhosis and advanced fibrosis, the study could not demonstrate a causal relationship between expression and disease.
The role of COX-2 in liver fibrosis is not fully understood, although several investigators have reported data from animal models demonstrating differential efficacy of COX-2 inhibitors in this disease. Tu et al. [28] reported that selective inhibition of COX-2 by rofecoxib in vivo reduced portal hypertension and was associated with antifibrotic activity. However, Hui et al. [23] demonstrated that celecoxib potentiated experimental liver fibrosis in rats, whereas Yu et al. [25] suggested that COX-2 did not appear to mediate the development of liver fibrosis in transgenic mice. The side effects of COX-2 inhibitors include potential renal, gastrointestinal, and cardiovascular events. Cardiovascular system safety is of particular importance, as COX-2 inhibitors reportedly can cause an increased risk of myocardial infarction [35]. Therefore, the US Food and Drug Administration has removed two of these agents (valdecoxib and rofecoxib) from the market. Additional investigations including an animal study to assess the safety of selective COX-2 inhibitors and their effects on the progression of fibrosis and development of HCC may be required.
In conclusion, COX-2 expression was significantly higher in cirrhosis compared with chronic hepatitis. When we categorized the all study tissues into low- and high-fibrosis stages, a significant association was observed between COX-2 expression and the extent of fibrosis. Thus, COX-2 may play a role in the progression of hepatic fibrosis, and further study including an additional animal study to investigate the effects of COX-2 inhibitors on hepatic fibrosis may be warranted.
Notes
No potential conflict of interest relevant to this article was reported.