Epidemiology of Ciprofloxacin Resistance and Its Relationship to Extended-Spectrum β-Lactamase Production in Proteus mirabilis Bacteremia
Article information
Abstract
Background/Aims
We evaluated the clinical features of ciprofloxacin-resistant Proteus mirabilis bacteremia and risk factors for ciprofloxacin resistance.
Methods
From October 2000 to July 2009, 37 patients with clinically significant P. mirabilis bacteremia were identified and data from patients with ciprofloxacin-resistant and ciprofloxacin-susceptible P. mirabilis bacteremia were compared.
Results
The most common underlying diseases were neurologic disease (37.8%) and solid tumors (29.7%). The most common site of infection was the urinary tract (35.1%). Ten of the 37 patients (27.0%) were infected with ciprofloxacin-resistant isolates, and univariate analysis revealed a significant relationship between ciprofloxacin-resistant P. mirabilis bacteremia and neurologic disease, recent operation, L-tube insertion, percutaneous tube use, and extended-spectrum β-lactamase (ESBL) production (all p < 0.05). ESBL was detected in six of 10 (60%) ciprofloxacin-resistant isolates, while only three of 27 (11%) ciprofloxacin-susceptible isolates produced ESBL (p = 0.005). In a logistic regression analysis, ESBL production remained a significant factor associated with ciprofloxacin resistance, after adjusting for other variables.
Conclusions
These data indicate a close association between ciprofloxacin resistance and ESBL-production in P. mirabilis bacteremia. This association is particularly troublesome because the therapeutic options for serious infections caused by ESBL-producing P. mirabilis are severely restricted.
INTRODUCTION
Proteus mirabilis can cause a variety of community- or hospital-acquired infections, including urinary tract, intraabdominal, and bloodstream infections [1,2]. Antimicrobial resistance has been reported increasingly for this species, and increased resistance to extended-spectrum cephalosporins due to the production of extended-spectrum β-lactamases (ESBLs) has become of great concern [1,3,4]. Consequently, carbapenem has emerged as the agent of choice for the treatment of serious infections caused by ESBL-producing pathogens. Fluoroquinolones may be an effective alternative antimicrobial therapy. However, similar to Escherichia coli and Klebsiella pneumoniae, the incidence of ciprofloxacin resistance in P. mirabilis isolates is increasing [5,6].
Although the occurrence of ciprofloxacin resistance in E. coli and K. pneumoniae isolates is well-established, little is known of the epidemiology of P. mirabilis bacteremia caused by ciprofloxacin-resistant isolates. Thus, in this study, we evaluated the clinical features of ciprofloxacin-resistant P. mirabilis bacteremia to clarify the risk factors for ciprofloxacin resistance in P. mirabilis isolates causing bacteremia.
METHODS
The database in our clinical microbiology laboratory (Samsung Medical Center, Seoul, Korea; a 1,950-bed, tertiary-care university hospital and referral center) was reviewed to identify patients > 16 years of age diagnosed with P. mirabilis bacteremia from October 2000 to July 2009. Only the first episode for each patient was included in the analysis. Antibiotic susceptibility was tested using the VITEK II automated system (bioMérieux, Hazelwood, MO, USA) using the standard modified broth microdilution method. Minimum inhibitory concentration (MIC) breakpoints and quality control protocols were used according to standards established by the Clinical and Laboratory Standards Institute [7]. For the purposes of this study, isolates showing in vitro resistance to cefotaxime, ceftriaxone, or ceftazidime were classified as ESBL-producing organisms. Strains showing an 'intermediate' result to the tested antibiotics were considered resistant.
We compared the clinical features of patients with ciprofloxacin-resistant and ciprofloxacin-susceptible P. mirabilis bacteremia. The data collected included age, gender, underlying disease, primary site of infection, acquisition site, and antimicrobial regimen. The presence of the following comorbid conditions was also documented: recent operation, corticosteroid use, indwelling urinary catheter, L-tube insertion, percutaneous tube use, prior invasive procedure, and prior use of antibiotics. Nosocomial acquisition of infection was defined as an infection that occurred > 48 hours after hospital admission, while infections diagnosed within the first 48 hours of hospitalization were classified as community-onset infection, based on the time the culture samples were obtained. Because many cases of Proteus bacteremia that are present or incubating on admission to the hospital are nonetheless healthcare-associated, we refer to non-nosocomial bacteremia as community-onset rather than community-acquired. Community-onset infection was further classified as community-associated and healthcare-associated, as previously suggested [8]. The site of infection was determined by physicians, based on the isolation of P. mirabilis from the presumed portal of entry and clinical evaluation.
Student's t test was used to compare continuous variables, and χ2 or Fisher's exact tests were used to compare categorical variables. A logistic regression analysis was performed to evaluate ESBL production as a factor associated with ciprofloxacin resistance. The odds ratio (OR) and 95% confidential interval (CI) were calculated. All p values were two-tailed, and p < 0.05 was considered to indicate statistical significance.
RESULTS
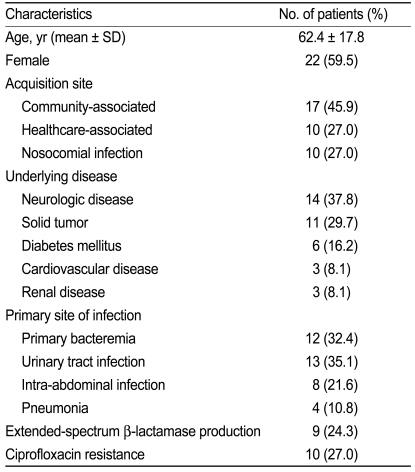
Thirty-seven patients with P. mirabilis bacteremia were identified. Their demographic and clinical characteristics are shown in Table 1. The most common underlying diseases were neurologic diseases (38%) and solid tumors (30%). The most common site of infection was the urinary tract (35%). Ten (27%) of the 37 patients were infected with ciprofloxacin-resistant isolates and nine (24%) were infected with ESBL-producers. Among the 27 patients determined to have community-onset infections, five (19%) had ciprofloxacin-resistant bacteremia. Among the ten patients identified as having a nosocomial-acquired infection, five (50%) had ciprofloxacin-resistant bacteremia (p = 0.094).
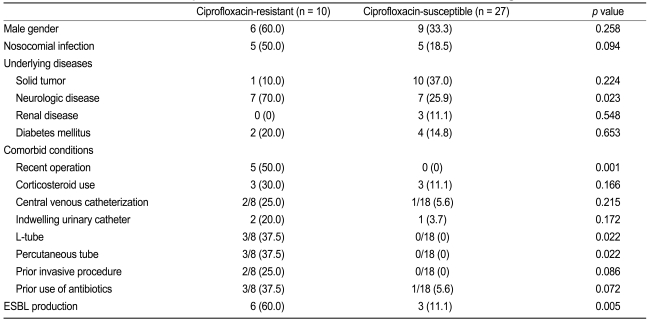
Risk factors associated with ciprofloxacin-resistant P. mirabilis are listed in Table 2. Univariate analysis revealed a significant relationship between ciprofloxacin-resistant P. mirabilis bacteremia and neurologic disease, recent operation, L-tube insertion, percutaneous tube use, and ESBL production (all p < 0.05). ESBL was detected in six (60%) of ten ciprofloxacin-resistant P. mirabilis isolates. In comparison, only three (11%) of 27 ciprofloxacin-susceptible P. mirabilis isolates were ESBL producers. ESBL production was significantly associated with ciprofloxacin resistance among P. mirabilis isolates causing bacteremia (OR, 12.00; 95% CI, 2.10 to 68.6; p = 0.005). In a logistic regression analysis, ESBL production remained a significant factor associated with ciprofloxacin resistance, after adjusting for nosocomial infection, neurologic disease, or recent operation (OR, 13.93; 95% CI, 1.04 to 186.07; p = 0.046).
Four (40%) of ten patients with ciprofloxacin-resistant bacteremia received inappropriate initial antimicrobial therapy, while three (11%) of 27 patients with ciprofloxacin-susceptible bacteremia received inappropriate initial antimicrobial therapy (p = 0.069). Of 32 patients whose outcomes could be evaluated, eight (25%) died within 30 days; no significant difference was found between ciprofloxacin-resistant (1/9, 11%) and -susceptible (7/23, 30%) patients (p = 0.386). When factors associated with mortality were evaluated, solid tumor was found to be the only significant factor associated with mortality (p = 0.005).
DISCUSSION
This study revealed that neurologic disease, recent operation, L-tube insertion, and percutaneous tube were significant risk factors associated with the development of bacteremia caused by ciprofloxacin-resistant P. mirabilis. Ciprofloxacin resistance in K. pneumoniae has been reported to be closely associated with ESBL production [9]. Several studies have examined the relationship between quinolone resistance and ESBL production in E. coli and K. pneumoniae [10,11]. Ciprofloxacin-resistant Enterobacter bacteremia is closely associated with broad-spectrum cephalosporin resistance [12]. Consistent with the latter study, we also observed a close association of ciprofloxacin-resistant P. mirabilis bacteremia with broad-spectrum cephalosporin resistance conferred by ESBL.
This association is of great concern because ESBL-producing P. mirabilis isolates are usually resistant to penicillins and cephalosporins. Thus, ciprofloxacin resistance severely limits already restricted treatment options. Additionally, the marked increase in the incidence of ESBL-producing P. mirabilis isolates in recent years is of great concern [1,3,13]. Although carbapenems remain the most effective option for treating serious infection caused by ESBL-producing P. mirabilis, the increasing use of carbapenems has been paralleled by the rapid emergence of carbapenem resistance in nosocomial pathogens [14,15].
What is the explanation for the coexistence of these two resistance mechanisms in Gram-negative bacilli? The relationship between ciprofloxacin resistance and ESBL production may be due to the interplay between prior heavy antibiotic use and conditions favoring patient-to-patient transfer of multidrug-resistant organisms, as described elsewhere [6,9,12]. Additionally, there are other potential explanations for the association between ciprofloxacin resistance and broad-spectrum cephalosporins resistance; these include active efflux and outer membrane protein alterations [16]. For ciprofloxacin resistance in K. pneumoniae and E. coli, transfer of the same plasmid has been suggested as a further possible explanation for the coexistence of the two resistance mechanisms [17,18]. Plasmid-mediated ciprofloxacin resistance has recently been reported in such strains [19,20]. Furthermore, a new plasmid-mediated quinolone resistance gene, qnrC, was reported in a clinical isolate of P. mirabilis [21]. However, the basis for the ciprofloxacin resistance combined with ESBL production is not yet fully understood. Further investigation of the possible mechanisms is needed.
Prior therapy with fluoroquinolones is an important risk factor for ciprofloxacin resistance in Gram-negative bacilli [22,23]. In this regard, we were unable to assess possible associations between ciprofloxacin resistance and prior use of antibiotics in this study because of the small number of cases. Additionally, we sought to delineate the clinical features of Proteus bacteremia, rather than focusing on microbiological analyses, and so did not characterize ESBL types. Thus, misclassification with regard to ESBL production may have been possible in several patients. Finally, because our study was conducted in a large tertiary-care hospital, the results may not necessarily be applicable to other institutions.
In conclusion, our investigation of risk factors for ciprofloxacin-resistant P. mirabilis bacteremia revealed a close association between ciprofloxacin resistance and ESBL production. This association is particularly troublesome because the therapeutic options for serious infections caused by ESBL-producing P. mirabilis are severely restricted.
Acknowledgements
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea (Grant No. A084063).
Notes
No potential conflict of interest relevant to this article was reported.