The role of rhinosinusitis in severe asthma
Article information
Abstract
The prevalence of asthma is approximately 5% to 10% in the general population. Of these, approximately 5% to 10% are severe asthmatics who respond poorly to asthmatic drugs, including high-dose inhaled steroids. Severe asthmatics have persistent symptoms, frequent symptom exacerbation, and severe airway obstruction even when taking high-dose inhaled steroids. The medical costs of treating severe asthmatics represent ~50% of the total healthcare costs for asthma. Risk factors for severe asthma are genetic and environmental, including many kinds of aeroallergens, β-blockers, and anti-inflammatory drugs. Gastroesophageal reflux disease and factors such as denial, anxiety, fear, depression, socioeconomic status, and alcohol consumption can exacerbate asthma. Rhinitis and asthma usually occur together. There is increasing evidence that allergic rhinitis and rhinosinusitis may influence the clinical course of asthma. This review discusses the role of rhinosinusitis in severe asthma.
INTRODUCTION
Asthma is a chronic inflammatory airway disease involving episodic breathlessness and wheezing with airway hyperresponsiveness to environmental stimuli [1-3]. The prevalence of asthma is about 5% to 10% in the general population. Of these, approximately 5% to 10% are severe refractory asthmatics who respond poorly to asthmatic drugs, including high-dose inhaled steroids [4-18]. Severe asthma is defined by the level of current clinical control and risks of uncontrolled asthma, which can result in frequent severe exacerbations (or death) and/or adverse reactions to medications and/or chronic morbidity (including impaired lung function or reduced lung growth in children). Severe refractory asthmatics have persistent symptoms, frequent symptom exacerbation, and severe airway obstruction, even when taking high-dose inhaled steroids. Patients who do not reach an acceptable level of control at step 4 of the Global Initiative for Asthma guidelines (reliever medication plus two or more controllers) are defined as having difficult to control asthma [3].
Severe asthma includes untreated severe asthma, difficult to treat severe asthma, and treatment-resistant severe asthma. The treatment-resistant severe asthma group includes the following [19]: 1) asthma for which control is not achieved despite the highest level of recommended treatment; refractory asthma and corticosteroid-resistant asthma, and 2) asthma for which control can be maintained only with the highest level of recommended treatment. These definitions help support the treatment of patients with asthma, including both the level of current clinical control and the risk of deterioration [19].
Rhinosinusitis is thought to play a causal role in difficult to control asthma. Clinical and experimental studies indicate that sinonasal inflammation can result in worsening of lower airway disease [20], potentially induced by postnasal drip, nasobronchial reflex, or inf lammatory mediators. Proper medical and surgical management of sinusitis in asthmatic patients is known to improve sinonasal and asthmatic symptoms with fewer physician visits and decrease the need for medication in several patients [21,22]. This review discusses the role of rhinosinusitis in severe asthma.
SEVERE ASTHMA
Severe asthma, including refractory asthma, represents 5% to 10% of asthma cases and is associated with more drug medication, hospital visits, and admissions than mild to moderate asthma. Mortality occurs in 3% to 35% of severe asthma cases, and the medical cost to treat severe asthma accounts for more than 50% of the total medical cost for treating asthmatic patients [23].
Risk factors for severe asthma are genetic and environmental, including many kinds of aeroallergens, β-blockers, and anti-inf lammatory drugs. Gastroesophageal ref lux disease (GERD) can affect asthma symptoms through esophagopharyngeal reflux and aspiration. Additional factors such as psychopathologies, socioeconomic status, and alcohol consumption can exacerbate asthma. Differential diagnoses [17] include smoking, chronic obstructive pulmonary disease, bronchiectasis, allergic bronchopulmonary aspergillosis, chronic infection, rhinosinusitis, vocal cord dysfunction, thyroid diseases, and inappropriate drug use [24,25].
RHINOSINUSITIS IN ASTHMA
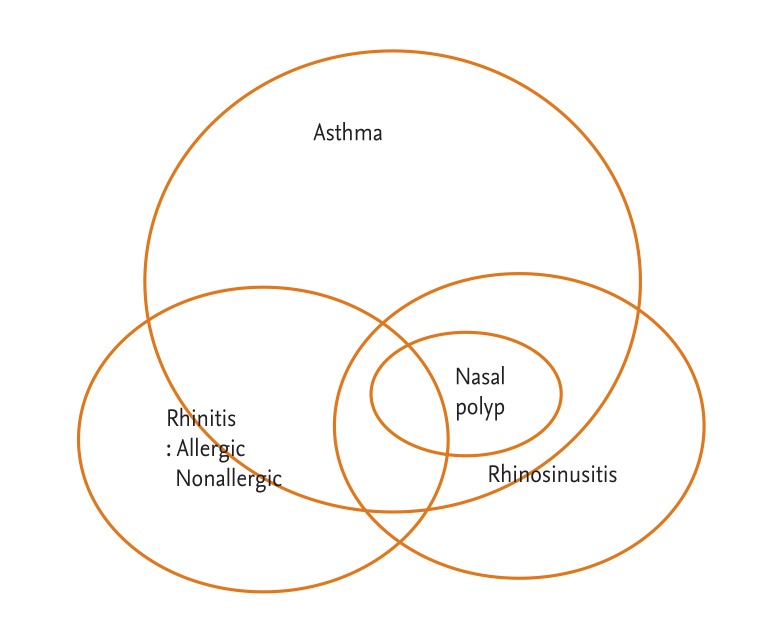
Allergic asthma and rhinitis are manifestations of the atopic syndrome and often coexist (Fig. 1). It is known that allergic rhinitis (AR) is a strong risk factor for the onset of asthma in adults [26]. There is increasing evidence of an association between AR and asthma based on epidemiological, immunological, and clinical studies [27]. Epidemiologically, up to 40% of patients with AR also have asthma, and up to 80% of patients with asthma experience nasal symptoms [27]. AR has been shown to increase the risk of asthma 3-fold [27]. Moreover, AR is linked to other comorbid conditions, including rhinosinusitis, nasal polyps, and otitis media with effusion [27].
Rhinitis and asthma usually occur together. There is increasing evidence that AR inf luences the clinical course of asthma. The prevalence of AR and asthma varies globally, with AR generally twice as prevalent as asthma [28]. Rhinitis is present in more than 80% of patients with allergic asthma [29]. Moreover, 76% of adult patients with AR and asthma reported the presence of rhinitis before the onset of asthma [29]. AR patients without symptoms of asthma often have bronchial hyperresponsiveness (BHR) to nonspecific bronchoconstrictors such as methacholine or histamine [30-35].
Several mechanisms have been proposed for the interaction between upper and lower airways in AR and asthma [30,36]. The direct effects are nasobronchial reflex, postnasal drip of inflammatory cells and/or mediators from the nose into the lower airways, and absorption of inflammatory cells and/or mediators from the nose into the systemic circulation and ultimately the lung [32,36]. The indirect effects are nasal obstruction causing reduction in filtration, humidification, and warming function of the nose [32,36]. AR and asthma are characterized by a similar inflammatory pattern in which eosinophils and T-lymphocytes are the predominant cells [28]. Eosinophilic inf lammation may be present in subjects with AR and BHR even in the absence of symptoms of asthma [37].
We retrospectively enrolled 1,492 asthmatics from the Cohort for Reality and Evolution of Adult Asthma in Korea cohort [38]. Asthmatics without atopy had more severe rhinitis compared with atopic asthmatics (severity [n = atopy/nonatopy], mild intermittent = 99/87 vs. moderate to severe intermittent = 59/35 vs. mild persistent = 232/197 vs. moderate to severe persistent = 68/83; p < 0.05).
Patients with severe asthma were older with longer disease durations, more daily symptoms, intense urgent health care utilization, sinusitis, and pneumonia, suggesting that severe asthma is characterized by abnormal lung function that is responsive to bronchodilators, a history of sinopulmonary infections, persistent symptoms, and increased health care utilization [39]. Aspirin sensitivity, GERD, sinusitis, and pneumonia were reported more often with severe asthma. The severe asthmatics reported more sinusitis history and requiring of surgical intervention [39].
Chronic rhinosinusitis (CRS) is characterized if two or more symptoms persist for greater than 12 weeks; symptoms include facial pain/pressure, purulent nasal discharge, nasal obstruction, and decreased sense of smell during chronic inflammation confirmed through endoscopy or radiographic studies [40,41]. CRS is a heterogeneous disorder comprised of two primary phenotypic presentations, clinically differentiated as either CRS with nasal polyposis or CRS without nasal polyposis [41]. Asthmatic patients had a higher rhinosinusitis severity score than nonasthmatic patients, and had more nasal polyps regardless of atopic status, indicative of a strong relationship between CRS severity and chronic airway inf lammatory diseases, asthma, and nasal polyps [42].
Symptomatic chronic sinusitis is an important comorbid condition in patients with asthma, both being associated with greater asthma severity [43-50]. Chronic sinusitis was also independently associated with more severe asthma [43] and was independently associated with moderate/severe asthma [43-50]. Both medical and surgical treatment of CRS was associated with subjective and objective improvements in asthma [43].
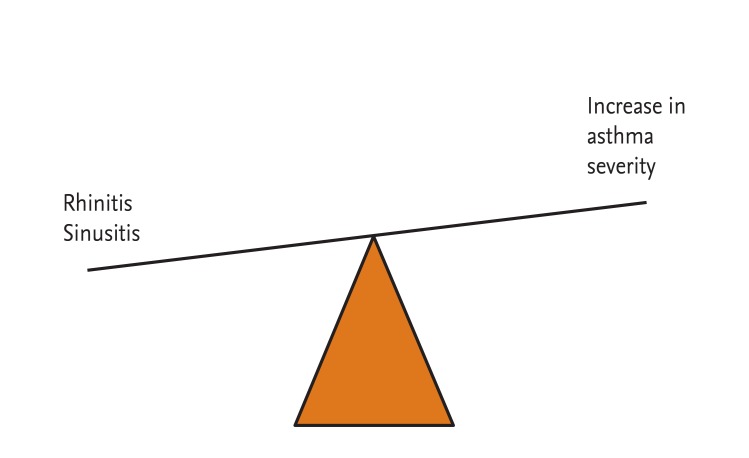
Chronic sinusitis is thought to play a causal role in difficult to control asthma [20]. Clinical and experimental studies indicated that sinonasal inflammation can result in worsening of lower airway disease [20], potentially induced by postnasal drip, nasobronchial reflex, or inflammatory mediators. The degree of rhinitis, as well as the presence of any or several signs of CRS, significantly increases the risk of having multisymptom asthma [51].
Proper medical and surgical management of sinusitis in the asthmatic patient has been shown to improve sinonasal and asthmatic symptoms with fewer physician visits and a decreased need for medication in several patients [18,21,22,52-54].
Successful management of asthma and rhinitis requires an integrated view of the airways, understanding of their interactions and an integrated treatment approach targeting systemic inflammation.
CONCLUSIONS
Severe asthmatics represent 5% to 10% of all asthmatics, but account for more than 50% of the total treatment costs of asthma. Rhinosinusitis can result in worsening of lower airway disease (Fig. 2). Proper medical and surgical management of rhinosinusitis in asthmatic patients results in improved sinonasal and asthmatic symptoms with fewer physician visits and a decreased need for medication. Understanding the pathophysiology of severe asthma and comorbidity, especially rhinosinusitis, is necessary for the development of effective therapeutics for severe asthma.
Notes
No potential conflict of interest relevant to this article is reported.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2013R1A1A2005465) and Soonchunhyang University Research Fund.