Is propofol safe when administered to cirrhotic patients during sedative endoscopy?
Article information
Abstract
Background/Aims
In patients with liver cirrhosis, drugs acting on the central nervous system can lead to hepatic encephalopathy and the effects may be prolonged. Recently, misuse of propofol has been reported and the associated risk of death have become an issue. Propofol is commonly used during sedative endoscopy; therefore, its safety in high-risk groups must be further investigated. We performed a pilot study of the safety and efficacy of propofol during endoscopy in Korean patients with cirrhosis.
Methods
Upper gastrointestinal endoscopy was performed under sedation with propofol along with careful monitoring in 20 patients with liver cirrhosis and 20 control subjects. The presence or development of hepatic encephalopathy was assessed using the number connection test and neurologic examination.
Results
Neither respiratory depression nor clinically significant hypotension were observed. Immediate postanesthetic recovery at 5 and 10 minutes after the procedure was delayed in the cirrhotic patients compared with the control group; however, at 30 minutes, the postanesthetic recovery was similar in both groups. Baseline psychomotor performance was more impaired in cirrhotic patients, but propofol was not associated with deteriorated psychomotor function even in cirrhotic patients with a minimal hepatic encephalopathy.
Conclusions
Sedation with propofol was well tolerated in cirrhotic patients. No newly developed hepatic encephalopathy was observed.
INTRODUCTION
Patients with liver cirrhosis are commonly referred for upper gastrointestinal (GI) endoscopy for the screening and treatment of complications of portal hypertension, such as esophagogastric varices and portal hypertensive gastropathy [1-3]. However, patients are often unwilling to undergo an endoscopic examination without proper sedation. Also, retching or vomiting during nonsedated endoscopic procedures may be associated with an increased risk of incidental variceal bleeding in these patients [4]. Although sedative endoscopy is now a common practice, the safety of the procedure in patients with liver cirrhosis is currently under debate [5]. One of the most frequent concerns is development of hepatic encephalopathy. Hepatic encephalopathy refers to a spectrum of neuropsychiatric abnormalities seen in patients with liver dysfunction after other brain diseases are excluded. It can be precipitated by narcotics, sedatives, opioids, other drugs acting on the central nervous system (CNS), hypotension, and hypoxemia [6].
Propofol (2, 6-diisoprophylphenol) is widely used for sedative endoscopy due to its rapid onset of action and faster recovery time [7,8]. However, it has some drawbacks, such as the possibility of apnea and hypotension, and there has been media attention paid to death related to propofol abuse. Therefore, the safety of this drug must be further evaluated in patients with liver cirrhosis. Several reports have demonstrated that the early pharmacokinetics of propofol are not significantly different between control and cirrhotic patients, and that propofol can be used even without major dose reductions [9,10].
Indeed, several studies have reported that propofol is relatively safe in cirrhotic patients [11-14]. However, most were performed in a Western population. In the current study, we aimed to evaluate the efficacy and safety of sedative endoscopy with propofol in Korean patients with liver cirrhosis.
METHODS
Study subjects
Patients who visited the Endoscopy Unit of Korea University Ansan Hospital were enrolled. The inclusion criteria for the cirrhotic patients group consisted of patients aged 20 to 65 years with liver cirrhosis. Diagnosis of liver cirrhosis was based on the results of liver biopsy or abdominal sonography along with compatible clinical history and laboratory findings. Patients who were over 65 years of age or had visual impairment, a history of hepatic encephalopathy or GI bleeding within a past month, active neurological impairment, any current psychiatric illness, symptomatic cardiopulmonary disease, or who were unwilling to participate were excluded. The control group consisted of patients undergoing GI endoscopy to evaluate dyspepsia who had no evidence of liver disease on clinical history or laboratory findings. The same exclusion criteria used for the cirrhotic patients was applied to the control group. We planned to perform this study as a pilot study, so the number of patients needed was not calculated statistically. The Institutional Review Board approved the study and informed consent was obtained from all patients.
Study design
In all the patients undergoing upper GI endoscopy, liver function was assessed according to the most recently obtained laboratory data. In cirrhotic patients, the Child-Turcotte-Pugh (CTP) score was estimated based on laboratory findings and physical examination. Before and after the endoscopic procedure, the number connection test (NCT) was performed in all patients with detailed explanation of the methods. Patients completed the questionnaire for educational level (elementary/middle/high school graduates, and college graduates or higher) before conducting the NCT, to correct the education-related bias [15]. The NCT involves documenting the time required to sequentially connect randomly placed numbers from 1 to 25, which has been proven to be suitable for diagnosing minimal or overt hepatic encephalopathy. Two different types of number arrangements were prepared for repetitive assessment, to eliminate learning effects. Examination of the presence of flapping tremor and NCT were performed twice, 20 minutes before and 40 minutes after the end of the endoscopic procedure. All procedures were performed by one endoscopist (H.J.Y.) to avoid interexaminer variability. Prophylactic oxygen was provided to all patients via a nasal cannula at a rate of 2 L per minute. Registered nurses then initiated propofol at 10 mg/kg in the cirrhotic patients group and 12 mg/kg in the control group via intravenous bolus followed by repeated injection of additional 1.0 to 1.5 mg/kg doses at 10 to 20 seconds intervals to achieve an appropriate level of sedation under supervision of the physician endoscopist. All medical staffs were certified in Advanced Cardiac Life Support. Baseline data were recorded for oxygen saturation, heart rate, and blood pressure throughout the procedure and in the recovery room. Oxygen saturation, heart rate, and an electrocardiogram were continuously monitored by pulse oximetry and telemetry. Blood pressure was measured once per minute during the procedure, and then every 5 minutes after the procedure until completion of the study. The Aldrete score, a postanesthetic recovery parameter assessing muscle activity, respiration, circulation, consciousness, and skin color [16], was recorded at 5, 15, and 30 minutes after the end of the procedure. A visual analogue scale (VAS), for which patients grade their level of pain during the endoscopy from point 0 (no discomfort) to 10 (severe pain), was conducted after the endoscopy to assess the efficacy of sedation. In the cirrhotic group, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin were followed-up 2 days after the endoscopic procedure to evaluate hepatic function after the use of propofol.
Statistical analyses
Data entry and statistical analysis were conducted using the SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA). Independent t test or Mann-Whitney U test was used to compare the mean age, total dose of propofol, procedure time, Aldrete score (at 5, 15, and 30 minutes), and preprocedural/postprocedural NCT score between the groups as appropriate. Paired t test or Wilcoxon signed rank test were performed to compare the preprocedural and postprocedural AST, ALT, and total bilirubin in the cirrhotic patients, and to compare the preprocedural and postprocedural NCT scores in cirrhotic patients and in controls. Educational level, sex ratio, and changes in oxygen saturation, blood pressure, and heart rate in the two groups were compared by chi-square test or Fisher exact test, as appropriate. All results with a two-sided p value of less than 0.05 were considered to be significant.
RESULTS
Baseline characteristics of the cirrhotic and control group
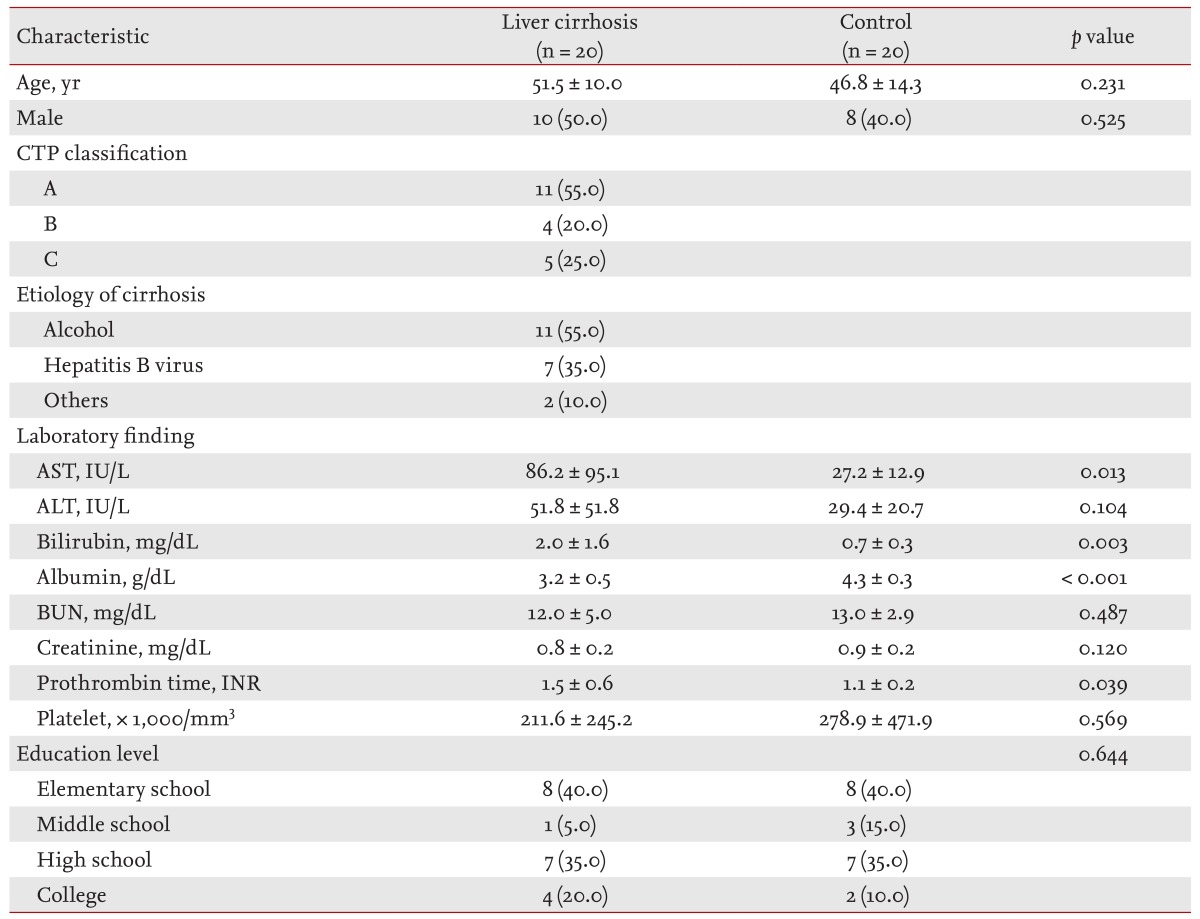
Twenty patients with liver cirrhosis (10 males, 10 females) and 20 control subjects (eight males, 12 females) were enrolled. The mean ages of cirrhotic patients and control subjects were 51.5 ± 10.0 years and 46.8 ± 14.3 years, respectively, which were not significantly different. The underlying causes of cirrhosis consisted of alcohol (55.0%) and chronic hepatitis B (35.0%). In addition, patients with primary biliary cirrhosis (5.0%) and cryptogenic cirrhosis (5.0%) were also included. The mean CTP score was 6.16 ± 2.4 (range, 5 to 10). Eleven patients (55.0%) were classified as CTP class A, four patients (20.0%) as CTP class B, and five patients (25.0%) as CTP class C. Mean age, gender, and educational levels did not differ in the two groups, as indicated by p values greater than 0.05 (Table 1). Moreover, most baseline biochemical tests, except serum AST, albumin, bilirubin, and prothrombin time (international normalized ratio), did not differ between the two groups. Table 2 shows the endoscopic findings in patients with liver cirrhosis. A total of 14 patients (70%) had esophageal varices, the size of which was classified according to the criteria of the Japanese Research Society [17]. An F1 varix was found in two patients (10.0%), F2 in nine patients (45.0%), and F3 in three patients (15.0%). Gastric varices were found in two patients (10%), a gastroesophageal varix was found in one patient, and portal hypertensive gastropathy was present in three patients (15.8%).
Efficacy and safety of sedation with propofol
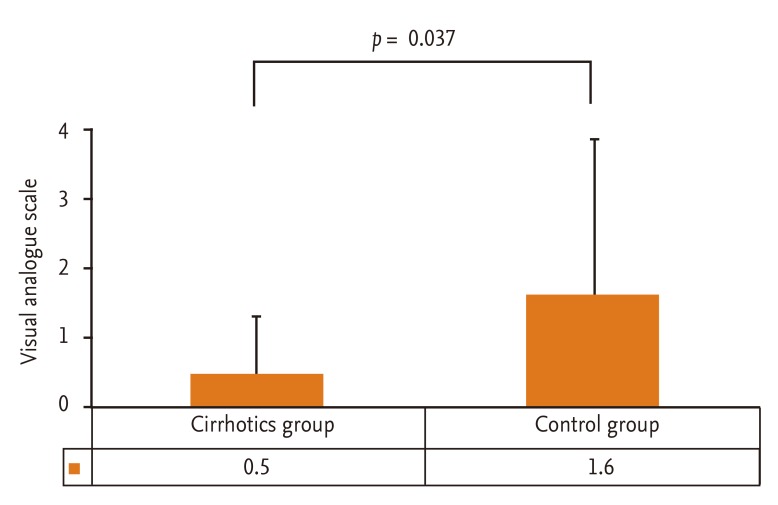
Total dose of propofol, procedure duration, hemodynamic changes, and Aldrete score are summarized in Table 3. Overall procedure durations were not significantly different between the two groups and were similar to those of previous studies (range, 4 to 6 minutes) [13]. The total dose for sedation was significantly lower in the cirrhotic patients group (p = 0.012). There were four hypotensive events (decrease of blood pressure > 20% below baseline), three in the cirrhotic patients group and one in the control group during or after the procedure; however, all hypotensive events were transient and recovered spontaneously within 1 to 2 minutes. Also, the frequency of such events was not different between the two groups (p = 0.109). There was one event of oxygen desaturation, which is defined as peripheral saturation decrease below 90%, in the control group, and the heart rate did not decrease more than 20% below the level of the baseline in either group. Aldrete score was 8.1 ± 0.9 versus 8.8 ± 0.9 at 5 minutes (p = 0.022), 9.0 ± 0.8 versus 9.5 ± 0.8 at 10 minutes (p = 0.049), and 9.8 ± 0.7 versus 9.9 ± 0.5 at 30 minutes (p = 0.609) in the cirrhotic and control groups, respectively, suggesting that immediate recovery was significantly delayed in the cirrhotic group; however, postanesthetic recovery at 30 minutes was similar between the two groups. The influence of propofol on hepatic function was evaluated by comparing the change in serum ALT and total bilirubin between the preprocedural level and postprocedural level measured 2 days after the procedure. The change in serum ALT, which indicates acute hepatocyte damage, was not significantly different in the cirrhotic group (51.8 ± 51.8 vs. 45.3 ± 35.9; p = 0.309). The total bilirubin showed a significant change (2.0 ± 1.6 vs. 1.6 ± 1.6; p = 0.012); however, the absolute mean value showed a minimal difference (Fig. 1). The efficacy of sedation with propofol assessed with VAS was significantly better in the cirrhotic group (p = 0.037) (Fig. 2). Corresponding to this result, most of the cirrhotic patients answered that they felt comfortable during and after the endoscopic procedure.
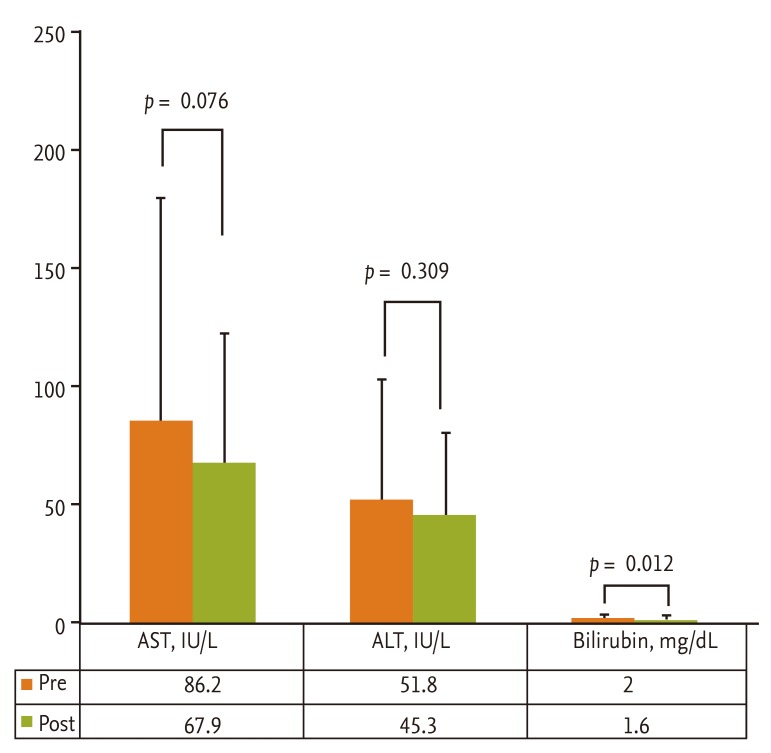
The levels of aminotransferases did not change signif icantly after the procedure in the cirrhotic group. Serum bilirubin levels were not elevated.
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Assessment of development of hepatic encephalopathy before and after sedative endoscopy with propofol
The mean times to NCT completion before the administration of propofol was 73.6 ± 42.8 seconds versus 45.0 ± 25.7 seconds in the cirrhotic and control groups, respectively (p = 0.015). The degree of the preprocedural NCT abnormality did not correlate with the CTP classification (r = 0.413, p = 0.070). Forty minutes after the completion of the procedure, the mean time for NCT was 71.5 ± 41.0 seconds versus 50.4±35.7 seconds, respectively, which tended to be longer in the cirrhotic group, albeit not significantly so (p = 0.089). Comparison of the mean time for NCT before and 40 minutes after the procedure showed no significant difference (p = 0.456 in the cirrhotic group and p = 0.172 in the control group) (Table 4).
Flapping tremor was not observed in either the preprocedural or postprocedural assessments in the cirrhotic and control groups.
Subgroup analysis according to liver cirrhosis etiology and liver function
According to the etiology of underlying liver diseases, patients were divided into the following two groups: alcohol-related (n = 11) and nonalcohol-related (n = 9) liver cirrhosis. AST, ALT, bilirubin, and albumin levels were not different between patients with alcoholic liver cirrhosis and those with nonalcoholic liver cirrhosis at the preprocedural and postprocedural assessments. The doses of propofol needed to achieve sedation in each group were 57.9 ± 11.5 and 69.7 ± 16.7 mg (p = 0.082), tending to be lower in patients with alcoholic cirrhosis, although there was no statistically significant difference. The Aldrete scores at 5, 10, and 30 minutes were 7.9 versus 8.1, 9.0 versus 8.9, and 9.6 versus 9.9 in alcoholic and nonalcoholic cirrhosis patients, respectively (p > 0.05 between the groups at all time points). NCT was not different at baseline and postprocedure in alcoholic cirrhosis (86.9 ± 56.4 vs. 83.6 ± 51.0; p = 0.445) and nonalcoholic (58.8 ± 16.7 vs. 59.3 ± 23.3; p = 0.887) cirrhosis patients. It was considered that etiology of underlying liver diseases did not influence on the outcome parameters.
According to the hepatic functional status, patients were divided into the following two groups: compensated (n = 11) and decompensated (n = 9) cirrhosis. AST, ALT, bilirubin, and albumin levels were not significantly different between the inpatients with compensated and those with decompensated cirrhosis at the preprocedural and postprocedural assessments. The doses of propofol needed for sedation were 62.5 ± 14.5 and 66.3 ± 17.3 mg, respectively (p = 0.605), and there was no significant difference. However, immediate postanesthetic recovery was delayed in decompensated patients; the Aldrete scores at 5, 10, and 30 minutes were 8.3 versus 7.4 (p = 0.024), 9.1 versus 8.7 (p = 0.321), and 9.7 versus 9.9 (p = 0.636) in compensated and decompensated cirrhosis patients, respectively. Nevertheless, the NCT was not different between the baseline and postprocedure timepoints in either the patients with compensated (62.6 ± 42.1 vs. 63.2 ± 38.0; p = 0.803) or decompensated (91.9 ± 40.6 vs. 86.7 ± 43.5; p = 0.437) cirrhosis.
Among the patients with decompensated cirrhosis, two had a history of hepatic encephalopathy over 1 month before enrollment. The total bilirubin level was significantly higher in patients with a history of hepatic encephalopathy (n = 2) than those without it (n = 18) at baseline (5.6 mg/dL vs. 1.8 mg/dL; p < 0.001) and postprocedure (5.5 mg/dL vs. 1.3 mg/dL; p = 0.001). However, other baseline and postprocedural parameters were not significantly different.
DISCUSSION
Variceal bleeding is one of the most fatal complications of portal hypertension in patients with liver cirrhosis [2,17]. Thus, time-appropriate upper GI endoscopy for the assessment of esophago-gastric varices and portal hypertensive gastropathy is important. Sedative endoscopy is currently a preferred method, but the drugs used for sedation during endoscopy may induce hepatic encephalopathy and impair psychomotor responses in cirrhotic patients [6].
Propofol, which is used for induction of anesthesia, is metabolized in the liver. Due to the absence of a significant difference in protein binding between patients with hepatic disease and healthy volunteers, there is no exaggerated pharmacological response, even in cirrhotic patients [9,10]. So, there is little need for propofol dose adjustment in cases of hepatic impairment, and it seems more useful in sedation of cirrhotic patients than any other drug [9,18]. However, propofol acts by releasing γ-aminobutyric acid (GABA) in the brain and activates the GABA receptor to induce depression of the CNS [7,8]. Thus it may lead to hepatic encephalopathy [19], and the full effects of propofol on the CNS must be further investigated.
The results of this study show that it took longer to complete NCT at baseline in the cirrhotic group compared with the control group, suggesting the possibility of minimal hepatic encephalopathy, which refers to patients who apparently show normal mental status on clinical examination but exhibit neurological impairment documented by neuropsychological tests [20,21]. It is a diagnosis of exclusion on the basis of other effects from confounding factors such as alcohol, drugs, or visual impairment [22]. Minimal hepatic encephalopathy is a highly prevalent disturbance, especially in patients with advanced liver cirrhosis [23]. Its prevalence in cirrhotic patients has been reported to vary from 30% to 84% [14,24,25]. These patients may be overtly asymptomatic, but attention deficits and slow information processing times may be present [26,27]. Several psychometric tests, such as the block design test, digital symbol test and the NCT, have been used to diagnose the condition [23,27]. Among these, the NCT is easiest to perform and is highly sensitive, and has the advantage that neuropsychiatric dysfunction can be expressed as a numerical value [27-29]. For these reasons, we used the NCT to detect minimal hepatic encephalopathy. Our results indicated no difference in the time required for the completion of NCT between preprocedure and postprocedure in the cirrhotic group (p = 0.456). It is thought that sedative endoscopy did not aggravate the minimal hepatic encephalopathy, in contrast to a previous report [19]. In addition, sedation with propofol did not affect liver function as assessed by biochemical laboratory tests, such as serum AST and ALT.
Our study is not the first on the safety of propofol use in diagnostic endoscopy. However, Korean data on propofol in the high risk group are still limited. Although misuse of propofol and associated death have been reported [30-32], this became a social issue in Korea recently. Thus, we were prompted to further evaluate the safety of propofol in cirrhotic patients who are thought to be vulnerable to sedatives or anesthetic drugs.
Distinguishably from a previous study that enrolled predominantly patients with relatively well-preserved liver function (CTP class A), we included more patients whose liver function was more decompensated (CTP class B or C). In our study, 45% of patients belonged to CTP class B or C as compared to 30% in the study by Correia et al. [13]. Also, the dose of propofol used here was higher compared to another study that described the effects of propofol (66.7 ± 16.3 mg vs. 57 ± 12 mg, respectively) in cirrhotic patients [14]. Although our patients had more decompensated liver function and received higher doses of propofol than in other studies, sedation with propofol was safe under proper monitoring. Compared to that by Yoon et al. [33] in Korea, the present study included patients with more advanced liver disease. Although the sample size in this study was small, we assessed safety and efficacy in a wide range of hepatic function in cirrhotic patients. In addition, we assessed the educational status of the patients to take into account its influence on the results of the NCT in the cirrhotic and control groups; the educational status of the two groups was well balanced.
We performed a subgroup analysis according to etiology and liver function. The etiology did not affect the outcomes although the efficacy of propofol has been reported to be decreased in frequent alcohol drinkers [34]. Also, the dose of propofol needed for sedation was not different between the alcoholic and nonalcoholic cirrhosis groups. The reason may be the fact that our patients had quit actively drinking at the time of enrollment due to their advanced liver disease.
The major limitation of our study was the small sample size. Thus a future larger study is needed to support our results because the number of patients did not provide sufficient statistical power. Nevertheless, our data are useful as reports on the efficacy and safety of propofol in Korean cirrhotic patients are limited.
In conclusion, sedation with propofol did not affect cognitive and psychomotor functions and was not related to the development of hepatic encephalopathy during upper GI endoscopy for the evaluation of complications in Korean cirrhotic patients. Also, propofol use was safe under proper monitoring and was effective for sedation in this population. Further studies are required to determine the optimal dose of propofol in more advanced cirrhotic patients and to evaluate its safety during therapeutic endoscopic procedures.
KEY MESSAGE
1. Sedation with propofol did not affect cognitive and psychomotor functions and was not related to the development of hepatic encephalopathy during upper gastrointestinal endoscopy to evaluate esophagogastric varices in Korean cirrhotic patients.
2. The usage of propofol was safe under proper monitoring and was effective for sedation.
Acknowledgments
This study was supported by a grant from the Korea Healthcare technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020).
Notes
No potential conflict of interest relevant to this article is reported.