Amlodipine and cardiovascular outcomes in hypertensive patients: meta-analysis comparing amlodipine-based versus other antihypertensive therapy
Article information
Abstract
Background/Aims
This meta-analysis compared the effects of amlodipine besylate, a charged dihydropyridine-type calcium channel blocker (CCB), with other non-CCB antihypertensive therapies regarding the cardiovascular outcome.
Methods
Data from seven long-term outcome trials comparing the cardiovascular outcomes of an amlodipine-based regimen with other active regimens were pooled and analyzed.
Results
The risk of myocardial infarction was significantly decreased with an amlodipine-based regimen compared with a non-CCB-based regimen (odds ratio [OR], 0.91; 95% confidence interval [CI], 0.84 to 0.99; p = 0.03). The risk of stroke was also significantly decreased (OR, 0.84; 95% CI, 0.79 to 0.90; p < 0.00001). The risk of heart failure increased slightly with marginal significance for an amlodipine-based regimen compared with a non-CCB-based regimen (OR, 1.14; 95% CI, 0.98 to 1.31; p = 0.08). However, when compared overall with β-blockers and diuretics, amlodipine showed a comparable risk. Amlodipine-based regimens demonstrated a 10% risk reduction in overall cardiovascular events (OR, 0.90; 95% CI, 0.82 to 0.99; p = 0.02) and total mortality (OR, 0.95; 95% CI, 0.91 to 0.99; p = 0.01).
Conclusions
Amlodipine reduced the risk of total cardiovascular events as well as all-cause mortality compared with non-CCB-based regimens, indicating its benefit for high-risk cardiac patients.
INTRODUCTION
Amlodipine, a charged dihydropyridine-type (DHP) calcium channel blocker (CCB), has been widely used to treat angina and hypertension. Several meta-analyses have evaluated the effect of CCBs on cardiovascular outcomes [1,2,3]. However, such analyses have usually been performed by merging the results from non-DHP CCBs with those from DHP CCBs, which have remarkably different characteristics [4]. A difference among DHP CCBs has even been suggested [5]. Of the CCBs, amlodipine is the most widely prescribed agent and has been the focus of the greatest number of studies [6], giving rise to this specific meta-analysis.
Here, the effects of amlodipine besylate on cardiovascular outcomes were evaluated in hypertensive patients. We performed a meta-analysis using data from large-scale, comparative, long-term outcome trials that evaluated the cardiovascular outcomes of amlodipine-based regimens. We also performed a subanalysis by dividing the studies into two groups. The first group consisted of studies that compared amlodipine-based regimens with non-CCB-based conventional regimens, including those using diuretics or β-blockers, and involved a meta-analysis of data from the ALLHAT/chlorthalidone [7], ACCOMPLISH [8], AASK/metoprolol [9], and ASCOT [10] trials. The second group consisted of studies that compared amlodipine-based regimens with renin-angiotensin system (RAS)-blocking regimens, including angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), and involved a meta-analysis of data from the AASK/ramipril, ALLHAT/lisinopril, IDNT [11], VALUE [12], and CASE-J [13] trials.
METHODS
Trial eligibility
This meta-analysis complied with the QUOROM statement [14]. Using PubMed searches of the MEDLINE database, we identified studies designed to evaluate the effects of amlodipine on the incidence of cardiovascular events in hypertensive patients. The study criteria for inclusion into the meta-analysis were: a randomized controlled trial published in a peer-reviewed journal, inclusion of patients with hypertension, comparison of amlodipine with another antihypertensive drug, assessment of cardiovascular events, follow-up of 1 year or longer, and a sample size of 100 or more. The search strategy was based on the search terms "stroke or myocardial infarction" or "coronary heart disease" or "heart disease" or "cardiovascular disease" and "hypertension" and "amlodipine." The searches were performed up to November 2011. All available English abstracts were reviewed, and the full text was consulted as necessary to clarify eligibility status. We excluded studies that examined only surrogate endpoints for cardiovascular disease (e.g., blood pressure lowering efficacy), studies with primary outcomes other than cardiovascular disease, and studies that excluded hypertensive subjects. We limited the data to the five most commonly used antihypertensive classes (diuretics, β-blockers, CCBs, ACE inhibitors, and ARBs).
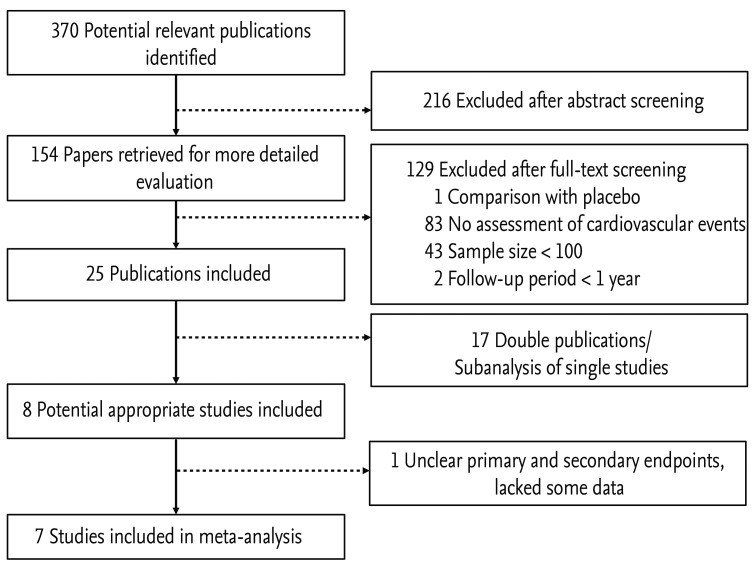
The initial search identified 370 articles; of these, eight met all of the inclusion criteria (Fig. 1). Among these, one trial was excluded (FACET) because it did not define clear primary and secondary endpoints, lacked some data, and, most importantly, was retrospective in design [15]. All eligible trials are listed in Table 1, which shows data on the number of events in each randomized group.
Comparisons and outcomes
For these analyses, the comparison studies were divided into two groups. The first group contained studies that compared amlodipine-based regimens with non-CCB-based conventional regimens such as diuretics (ALLHAT/chlorthalidone and ACCOMPLISH) or β-blockers (AASK/metoprolol and ASCOT). The second group contained studies that compared amlodipine-based regimens with RAS-blocking regimens such as ACE inhibitors (AASK/ramipril and ALLHAT/lisinopril) or ARBs (IDNT, VALUE, and CASE-J).
The data were abstracted and differences were resolved by consensus. Data on the outcomes of myocardial infarction, stroke, congestive heart failure (CHF), major cardiovascular events, total mortality, and cardiovascular disease mortality were analyzed. The major fatal and nonfatal cardiovascular events included myocardial infarction, stroke, and CHF. The major cardiovascular events included coronary heart disease (CHD), stroke, CHF, and other cardiovascular disease mortalities. While the definition of endpoints varied slightly among the trials, the endpoint definitions and methods of classification were identical across the treatment groups within each trial.
Statistical analyses
Statistical calculations and graphs were produced using Cochrane Review Manager (RevMan) software, ver. 5.0 (Cochrane Collaboration, Oxford, UK). Two-tailed statistical significance was set at 5%, except for the Cochran's chi-square test for heterogeneity, which used a 10% level of significance. The Dersimonian-Laired method was used for the random-effects model. The pooled results for each outcome are presented as odds ratios (ORs) with 95% confidence intervals (CIs).
Before applying approximate chi-square tests for heterogeneity, we assessed the studies for heterogeneity in patient characteristics, interventions, and outcomes. Statistical heterogeneity was also examined with I2 statistics, where I2 values ≥ 50% were considered to be indicators of a substantial level of heterogeneity. Forest plots were also used for visual inspection. Funnel plots of effect estimates against the standard error were examined to assess publication bias.
RESULTS
The seven eligible studies included 87,257 patients. The baseline characteristics of the seven trials included in the meta-analysis are shown in Table 2. The mean duration of follow-up was 4.6 years (range, 3 to 6 years). The mean age of the trial patients was 66 years and 31% were women. About 39% of the patients had diabetes mellitus and 18% were smokers.
Myocardial infarction
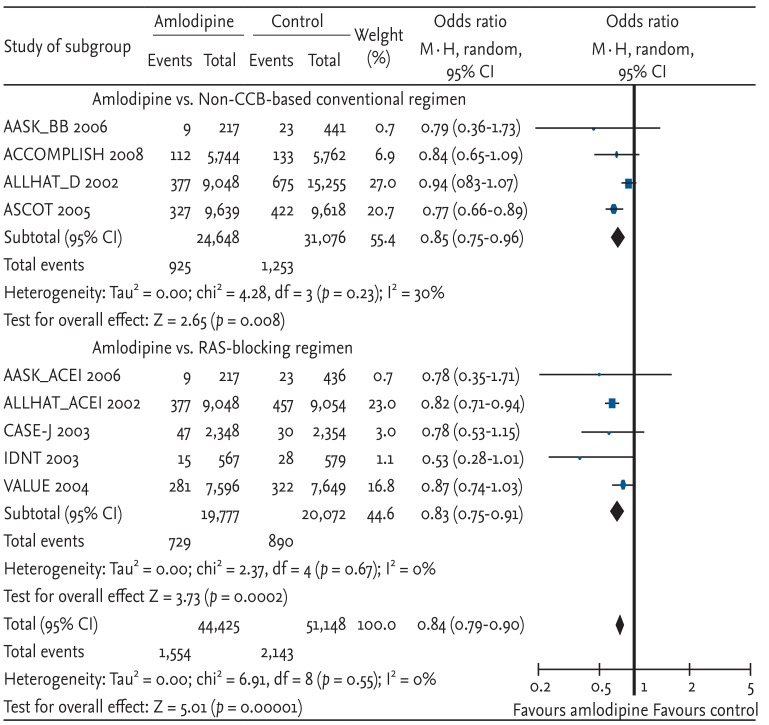
The risk of myocardial infarction was significantly decreased with amlodipine-based regimens compared with other antihypertensive drugs (OR, 0.91; 95% CI, 0.84 to 0.99; p = 0.03; heterogeneity p = 0.13) (Fig. 2). The risk reduction of amlodipine-based therapy was similar to that of the non-CCB-based regimen, although neither reached statistical significance due to a decreased sample size. Each study result, except CASE-J and ALLHAT (comparisons of amlodipine with an ACE inhibitor), showed similar favorable results for amlodipine-based regimens.

Effects of amlodipine on myocardial infarction in trials comparing other antihypertensive drugs [7,8,9,10,11,12,13]. Blue squares represent treatment-to-control odds ratios in the trials; their size is proportional to the number of events. The 95% confidence intervals (CIs) for individual trials are denoted by lines, while those for pooled odds ratios are denoted by diamonds. CCB, calcium channel blocker; RAS, renin-angiotensin system.
Stroke
Amlodipine provided better protection against stroke compared with non-CCB-based conventional regimens and with RAS-blocking regimens. As a result, the risk of stroke was significantly decreased with amlodipine-based regimens compared with other antihypertensive drugs (OR, 0.84; 95% CI, 0.79 to 0.90; p < 0.00001; heterogeneity p = 0.55) (Fig. 3). The individual study results showed a similar range of protection with amlodipine.
Heart failure
The risk of heart failure seemed to increase with marginal significance with amlodipine-based regimens compared with other antihypertensive drugs (OR, 1.14; 95% CI, 0.98 to 1.31; p = 0.08; heterogeneity p = 0.0008, Fig. 4). An analysis of the overall results showed an inferior effect of amlodipine-based regimens compared with RAS-blocking regimens (OR, 1.19; 95% CI, 1.03 to 1.37; p = 0.02; heterogeneity p = 0.16). However, when compared with non-CCB-based conventional regimens, amlodipine-based regimens showed a comparable effect (OR, 1.04; 95% CI, 0.75 to 1.44; p = 0.82; heterogeneity p = 0.0002). Among the trials comparing conventional therapies, ALLHAT-diuretics (chlorthalidone) were significantly more effective than amlodipine-based regimens, whereas other trials, including ACCOMPLISH with hydrochlorothiazide, showed no significant difference compared with amlodipine-based regimens.
Combined major cardiovascular events
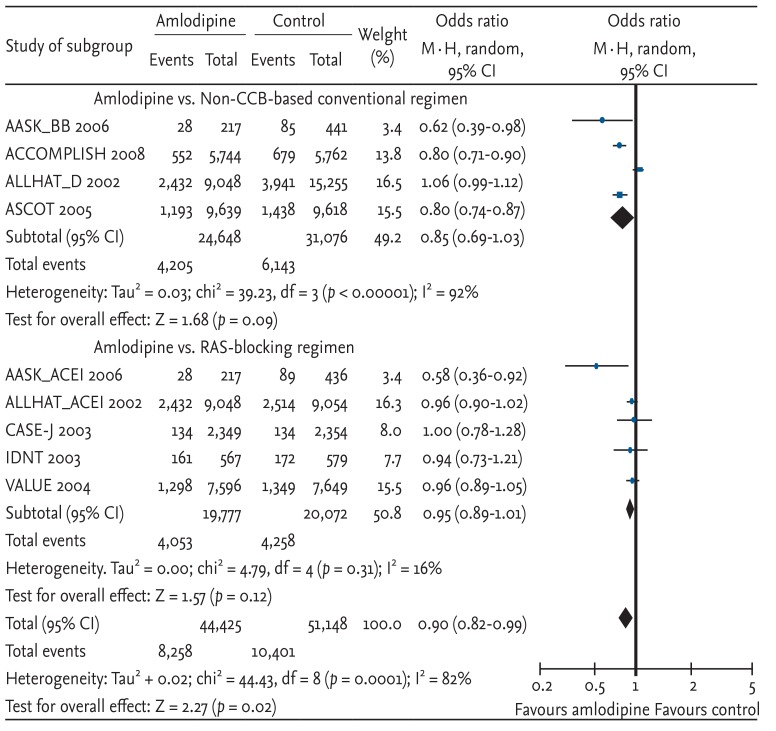
Cardiovascular disease events, which combined CHD, stroke, CHF, and other cardiovascular disease mortalities, were compared. Amlodipine-based regimens showed a 10% risk reduction, which was statistically significant (OR, 0.90; 95% CI, 0.82 to 0.99; p = 0.02; heterogeneity p < 0.00001) (Fig. 5). In terms of the absolute value of risk reduction, the OR for amlodipine-based regimens seemed to show lower risk compared with non-CCB-based conventional regimens (OR, 0.85) or RAS-blocking regimens (OR, 0.95). However, neither sub-analysis was statistically significant.
Total and cardiovascular mortality
Lastly, we compared the risk of total and cardiovascular mortality on an amlodipine-based regimen with that on other antihypertensive drugs. Amlodipine-based regimens demonstrated a significant risk reduction compared with other antihypertensive drugs (OR, 0.95; 95% CI, 0.91 to 0.99; p = 0.01; heterogeneity p = 0.70) (Fig. 6A). The extent of risk reduction was greater when compared with non-CCB-based conventional regimens (OR, 0.93; 95% CI, 0.88 to 0.98; p = 0.01; heterogeneity p = 0.72). The risk was not increased when compared with RAS-blocking regimens (OR, 0.97; 95% CI, 0.91 to 1.03; p = 0.37; heterogeneity p = 0.55).
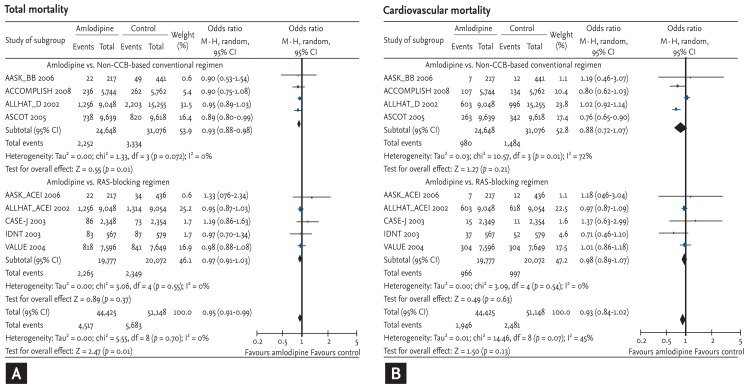
Effects of amlodipine on total and cardiovascular mortality in trials comparing other antihypertensive drugs [7,8,9,10,11,12,13]. (A) Total mortality. (B) Cardiovascular mortality. CI, confidence interval; CCB, calcium channel blocker; RAS, renin-angiotensin system. See Figure 2 for additional details.
The tendency was the same in cases of cardiovascular mortality. However, due to fewer events (4,427 cardiovascular mortality vs. 10,200 total mortality), there was no statistical significance (Fig. 6B).
DISCUSSION
This meta-analysis of seven large-scale, actively controlled, long-term outcome trials that included 87,257 patients shows that amlodipine-based regimens reduced the risk of myocardial infarction and stroke compared with non-CCB antihypertensive therapy. Amlodipine-based regimens reduced the risk of total cardiovascular events by 10%, and, surprisingly, significantly reduced the risk of total mortality compared to non-CCB antihypertensive therapies. For the most part, the results of our study are consistent with those of a meta-analysis of 27 overall CCB studies, which was performed before the ACCOMPLISH study was published [1]. However, the current study has several unique features that distinguish it from the previous meta-analysis.
First, this study analyzed the effect of a single DHP CCB, amlodipine. We intended to minimize the confounding effect of variability of treatment due to the substantial difference between DHPs and non-DHP CCBs [16]. This approach was possible since amlodipine was predominantly used as either the treatment regimen or the control regimen in more than half of the overall outcome studies with a CCB arm [1].
Second, this meta-analysis was limited to data from actively controlled trials to minimize the effect of blood pressure reductions observed in placebo-controlled trials. Although there were still blood pressure differences of up to 2.1 mmHg, which were lower in the amlodipine arms, the difference in blood pressure in the enrolled trials was much less than that in other meta-analyses, including placebo-controlled trials.
Lastly, we enrolled only trials that evaluated long-term outcomes, and thus had clearly defined primary and secondary endpoints. As most long-term outcome studies enrolled a far larger number of patients than small-scale intermediate endpoint studies, the current study avoided selection bias.
Since the six outcome trials analyzed in this study, except the ACCOMPLISH trial, were also included in a previous meta-analysis of 27 overall CCB studies that was published before the ACCOMPLISH study [1], we assumed that the risk reduction in total mortality and stroke by DHP CCBs would be reproduced in this meta-analysis. However, surprisingly, this study showed a significant risk reduction in myocardial infarction as well as composite cardiovascular events, which was not significant in the previous meta-analysis that combined the results of all CCBs, including non-DHP CCBs. There are several possible explanations for this difference.
The previous meta-analysis of CCBs showed a lack of protection against myocardial infarction (OR, 1.00; 95% CI, 0.95 to 1.04; p = 0.83) [1]. However, there were significant heterogeneities among the individual study results. In this study, an amlodipine-based regimen showed a significant risk reduction of 9%, which was consistent across the enrolled studies (OR, 0.91; 95% CI, 0.84 to 0.99; p = 0.03). Although it is debatable whether certain classes of antihypertensive agents are more effective than others, several papers have suggested that ACE inhibitors more effectively prevent coronary artery disease than CCBs. For example, a meta-analysis performed by Verdecchia et al. [17] reported a nonsignificant reduction in coronary artery disease risk by CCBs compared with a placebo. However, the results were largely heterogeneous for the enrolled studies. In this aspect, the significant risk reduction of 9% for myocardial infarction observed in this study did not contradict previous studies, but rather reflects homogeneity among studies that might be related to a single agent, amlodipine. The significant risk reduction in myocardial infarction by amlodipine is consistent with intermediate endpoint study results showing the antiatherosclerotic effect of DHP CCBs [18] and, specifically, the effect of amlodipine [19].
A second noteworthy finding regarded stroke prevention. It is widely accepted that CCBs are superior to other classes of antihypertensive agents for stroke prevention [20]. However, it remains debatable whether the protective effects are due to a reduction in blood pressure or to the ancillary effects of CCBs [21]. Our study revealed a significant risk reduction of 15%, which is also consistent with the overall CCB meta-analysis. Notably, because this meta-analysis confined the data from an active-controlled trial, there were blood pressure differences less than 2.1 mmHg between the amlodipine-based regimens and other antihypertensive arm. Moreover, the amlodipine-based regimens showed a risk reduction of approximately 15% regardless of the counterpart regimen, whether it was a non-CCB-based conventional regimen (e.g., diuretics and β-blockers) or a RAS-blocking regimen (e.g., ACE inhibitors and ARBs), which supports the 'beyond blood pressure lowering' effect of amlodipine. The mechanism behind stroke prevention by CCBs remains to be clarified. However, the longer duration of efficacy of CCBs compared with other classes might enable better blood pressure control in the early morning, which is considered the high-risk period for stroke [22]. In addition, it was recently suggested that the reduced blood pressure variation achieved with CCBs as compared with other classes of antihypertensives provides greater stroke prevention [23].
Perhaps the most striking result of this study regarded heart failure prevention. An overall CCB meta-analysis reported that the risk of heart failure was increased by CCB-based regimens compared with non-CCB treatments by 17% with substantial heterogeneity among studies [1]. The inferiority of CCBs was confirmed in this meta-analysis, which compared the effect of amlodipine with that of RAS-blocking agents such as ACE inhibitors or ARBs. In contrast, amlodipine-based regimens showed comparable results versus non-CCB-based conventional regimens such as diuretics or β-blockers. This comparability was obscured in a previous meta-analysis, which reported that overall CCBs, including non-DHP CCBs, were inferior, even to conventional regimens [1]. There was considerable heterogeneity in the effect among studies, including for conventional treatments. Only the ALLHAT-diuretics arm showed a far superior risk reduction by diuretics compared to amlodipine-based regimens. The incidence of CHF adjudication may have been overestimated in the case of CCBs due to ankle edema, a well-known adverse effect of CCBs that is unrelated to CHF. In contrast, CHF incidence might be underestimated in the diuretic arm because fluid retention caused by CHF is relieved by diuretics. This underestimation/overestimation may have been particularly substantial in the ALLHAT trial, which did not adjudicate heart failure as endpoints before study initiation [7]. Conversely, in the ACCOMPLISH trial, which classified heart failure as that requiring hospitalization, there was no significant difference (100 events in 5,744 patients vs. 96 events in 5,762 patients; OR, 1.04; 95% CI, 0.79 to 1.38; p = 0.77) between amlodipine- and hydrochlorothiazide-based regimens [8]. Although we accept the suggestion that ACE inhibitors and ARBs are superior to CCBs in heart failure prevention, the observation that CCBs are inferior to all other classes of antihypertensives cannot be over-interpreted, not only because CCBs showed an invariable risk reduction for heart failure by 28% compared with a placebo [1] but also because the heart failure incidence was comparable between the amlodipine-based regimens and non-CCB-based conventional regimens, as shown in this meta-analysis.
Lastly, amlodipine-based regimens showed an invariable reduction in total cardiovascular events of 10% and reduction in total mortality of 5%. The only exception was the ALLHAT-diuretics study, which showed a far superior reduction in heart failure incidence, as mentioned before. Although the risk reduction in cardiovascular mortality was not significant (OR, 0.93; 95% CI, 0.84 to 1.02; p = 0.13), this lack of significance could have been due to fewer events.
This study has some limitations. Although we included all long-term outcome trials, small-scale intermediate endpoints studies were excluded, possibly resulting in deviation of the meta-analysis. The definition of cardiovascular events, especially the definition of CHF, differed between the trials. Due to limitations on our ability to access data from individual patients, we did not perform a multivariable analysis, including potential effect modifiers such as mean age, sex, cigarette use, and previous cardiovascular diseases, which could have caused bias. Finally, all of the trials included in this meta-analysis used amlodipine besylate; therefore, we cannot speculate whether the results of this study can be extrapolated to other amlodipine salt formulations.
In conclusion, our meta-analysis showed a significant reduction in total cardiovascular events by 10% and in total mortality by 5% for amlodipine compared with non-CCB antihypertensive therapies, which had not been demonstrated previously in other meta-analyses of CCBs. We also identified a protective effect against myocardial infarction and stroke. CHF incidence seemed to be increased with amlodipine compared with ACE inhibitors or ARBs, but was comparable to that with β-blockers and diuretics. Therefore, amlodipine can be safely used in high-risk cardiac patients and is associated with benefits for all major cardiovascular endpoints as well as total mortality.
KEY MESSAGE
1. Amlodipine-based regimens reduced the risk of total cardiovascular events and mortality compared with noncalcium channel blocker antihypertensive therapies.
Notes
The data in this study were presented on a poster at the 76th annual scientific meeting of the Japanese Circulation Society, which was held March 16-18, 2012, in Fukuoka, Japan.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea (A070001; to Hae-Young Lee). The statistical analyses were supported by Hye-Jin Park and Su-Kyoung Ko of Pfizer Pharmaceuticals Korea, Ltd. The corresponding author received no honorarium/fee or other form of financial support related to the development of this article. We requested English editorial assistance from Harisco (http://www.harisco.net).
Notes
Hye-Jin Park and Su-Kyoung Ko are statisticians and employees in Pfizer Pharmaceuticals Korea, Ltd.