Follicular and Hurthle cell carcinoma of the thyroid in iodine-sufficient area: retrospective analysis of Korean multicenter data
Article information
Abstract
Background/Aims
Follicular thyroid carcinoma (FTC) and Hurthle cell carcinoma (HCC) of the thyroid are relatively uncommon thyroid malignancies in iodine-sufficient areas. In this study we evaluated the clinical behavior, prognostic factors and treatment outcomes of FTC and HCC in Korea.
Methods
This multicenter study included 483 patients with FTC and 80 patients with HCC who underwent an initial surgery between 1995 and 2006 in one of the four tertiary referral hospitals in Korea. We evaluated clinicopathological factors associated with distant metastases and recurrence during a median of 6 years of follow-up.
Results
HCC patients were significantly older (49 years vs. 43 years; p < 0.001) and had more lymphovascular invasions (22% vs. 14%; p = 0.03) compared with FTC patients. Distant metastases were confirmed in 40 patients (8%) in the FTC group and in two patients (3%) in the HCC group (p = 0.07). Distant metastases were significantly associated with older age, widely invasive cancer and extrathyroidal invasion. Only 14 patients (3%) had recurrent disease and there was no significant difference between FTC and HCC groups (p = 0.38). Recurrence was associated with larger tumor size and cervical lymph node metastasis.
Conclusions
HCC patients were older and had more lymphovascular invasions than FTC patients. However, FTC and HCC patients had similar initial clinicopathological features. Older age, wide invasiveness and extrathyroidal invasion were independent risk factors for predicting distant metastases in FTC and HCC patients.
INTRODUCTION
Follicular thyroid carcinoma (FTC) is the second most common type of thyroid cancer, accounting for 10% to 15% of all thyroid malignancies [1,2]. FTC is more common in areas of iodine-deficient endemic goiter and more prevalent in African Americans than in Asians or Caucasians [3]. Previously, FTC was considered a more aggressive type of cancer, as compared to papillary thyroid cancer (PTC), because more distant metastases occurred in FTC patients [4,5]. However, the prognosis of FTC and PTC patients is similar if their age and initial cancer staging is similar [6,7]. Traditionally, FTC was classified as either widely or minimally invasive [8]. The World Health Organization (WHO) has defined widely invasive FTC (WI-FTC) as a tumor with widespread infiltration of blood vessels and/or adjacent extrathyroid tissue and lacking complete encapsulation [9]. Minimally invasive FTC (MI-FTC) is a grossly encapsulated solitary tumor with unequivocal vascular invasion and/or invasion that penetrates the full thickness of the capsule [9]. Distant metastases are common in patients with WI-FTC, but rare in MI-FTC patients [10].
Hurthle cell carcinoma (HCC), also known as oncocytic or oxyphillic carcinoma is classified by the WHO as a variant of FTC [9,11]. However, recent genome-wide analysis using mutation genotyping suggested that HCC may be a unique thyroid cancer distinct from PTC and FTC [12]. Hurthle cells (oxyphilic cells) are large polygonal cells with distinct borders derived from thyroid follicular epithelium and contain abundant granular cytoplasm because of an excess of mitochondria and a large nucleus with a prominent nucleolus [13]. HCC is defined as a thyroid cancer consisting of at least 75% Hurthle cells and the presence of capsular and/or vascular invasion and can be classified into a minimally or widely invasive type [9].
Data on clinicopathological features and clinical outcomes in patients with FTC or HCC in iodine-sufficient areas are limited in Korea. In this multicenter study we evaluated the clinical behavior and prognostic characteristics of FTC and HCC in Korea. We compared initial pathological parameters and disease-free survival (DFS) between the minimally invasive and widely invasive cancers. We also described the preoperative cytological diagnosis using fine-needle aspiration (FNA) in patients with FTC or HCC.
METHODS
Subjects
A total of 563 patients with the histological diagnosis of FTC or HCC who underwent thyroid surgery at one of the four major referral centers in Korea (Asan Medical Center, Seoul National University Hospital, Samsung Medical Center, and Chungnam National University Hospital) between 1995 and 2006 were enrolled in the present study. Four hundred eighty-three patients with FTC and 80 patients with HCC underwent initial thyroid surgery during the study period. The study protocol was approved by the Institutional Review Board of the Asan Medical Center.
Clinicopathological parameters
The medical records of FTC and HCC patients were reviewed. The results of preoperative FNA cytology (FNAC) were evaluated. The results were categorized as benign, atypical cell, indeterminate, follicular neoplasm, Hurthle cell neoplasm, suggestive medullar thyroid cancer, suggestive PTC, PTC, poorly differentiated thyroid cancer (DTC), and nondiagnostic. Demographic data, pathological findings (tumor size, multifocality, lymphovascular invasion, extrathyroidal extension, cervical lymph node [LN] metastasis), and initial treatment modality were analyzed as potential prognostic factors. The distant metastasis in this study was defined as presence of distant metastasis at the time of initial therapy.
MI-FTC or HCC was defined as tumors with focal capsular invasion only. WI-FTC or HCC included tumors with angioinvasion or any extrathyroidal invasion. Follow-up data regarding recurrences were obtained from medical records at each center and recurrence was defined as the reappearance of disease after initial therapy, and confirmed by cytological or histological results.
Statistics
Continuous variables were expressed as means ± standard deviation values. Student t test was used for comparison of parametric variables between groups. Categorical variables were presented as numbers and percentages and compared using chi-square or Fisher exact test. The Kaplan-Meier method with the log-rank test was used to compare DFS between groups. The Cox proportional hazard model and the backward elimination method were used to analyze various prognostic factors for distant metastasis and recurrence. p values were two-sided throughout and p values less than 0.05 were considered statistically significant. Data were analyzed using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinicopathological characteristics
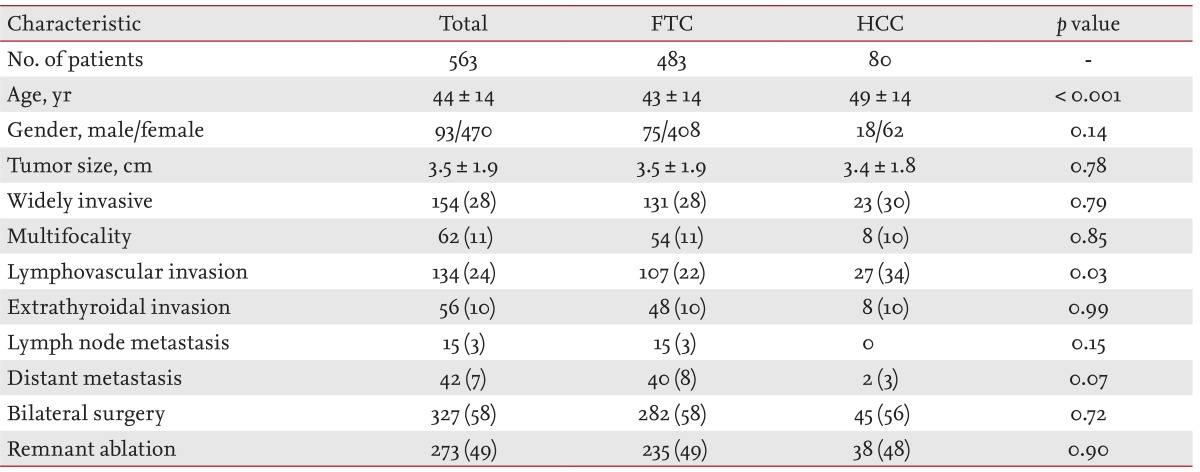
A total of 563 patients (93 males and 470 females; mean age, 44 ± 14 years) with FTC or HCC who underwent thyroid surgery were identified by our study protocol. HCC patients were significantly older than FTC patients (p < 0.001). However, significant differences in gender, tumor size and frequencies of multifocality, extrathyroidal invasion, or cervical LN metastasis were not observed between FTC and HCC patients (Table 1). HCC patients had more lymphovascular invasions than FTC patients (p < 0.03) and the prevalence of widely invasive cancer was similar between the FTC and HCC groups (p = 0.79). A similar proportion of patients in the two groups were treated with bilateral thyroidectomy and I-131 remnant ablation.
Preoperative FNAC results
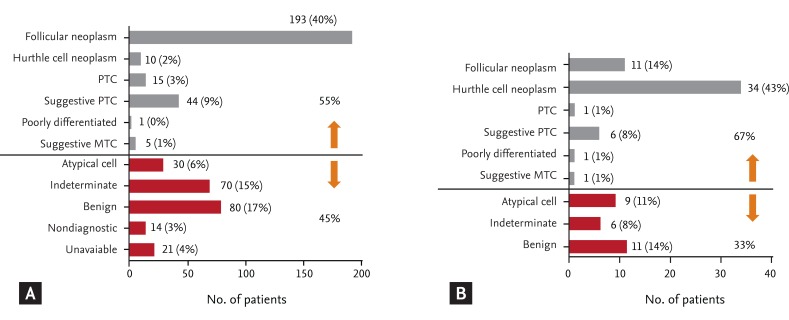
Cytological diagnosis after FNAC was available for the majority of patients (Fig. 1). Among 483 patients with FTC, 193 patients (40%) were diagnosed as follicular neoplasm and 10 patients were diagnosed as Hurthle cell neoplasm after FNAC (Fig. 1A). Sixty-five patients (13.4%) underwent thyroid surgery after cytological diagnosis of other types of thyroid cancer and their pathological diagnosis was FTC. Among 80 patients with HCC, the preoperative FNAC showed Hurthle cell neoplasm in 34 patients (43%) and follicular neoplasm in 11 patients (14%) (Fig. 1B). Nine of 80 patients (11%) also underwent thyroid surgery after cytological diagnosis of other types of thyroid cancer. Approximately 41% of FTC patients and 33% of HCC patients had non-malignant results such as atypical, indeterminate and benign cells based on their preoperative FNAC.
Clinicopathological parameters associated with distant metastasis
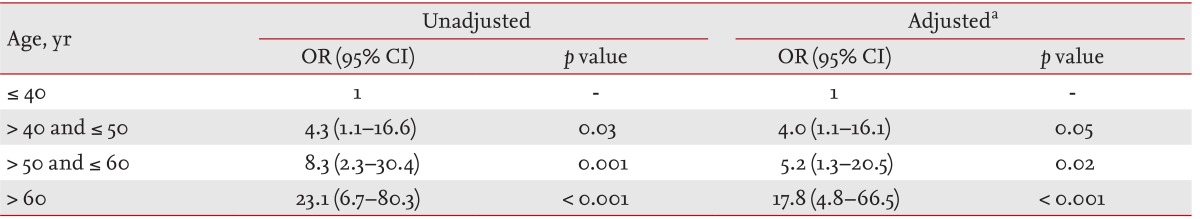
Among 563 patients, 42 (7%) had distant metastases as shown in Table 1. The difference in prevalence of distant metastases between FTC and HCC patients was marginally significant (p = 0.07). Older age, larger tumor size, widely invasive disease, lymphovascular invasion, extrathyroidal invasion, and cervical LN metastasis were significantly associated with distant metastasis in FTC and HCC patients (Table 2). Distant metastases were found more in patients who underwent bilateral thyroid surgery and I-131 remnant ablation. However, older age, widely invasive disease, extrathyroidal invasion and I-131 remnant ablation showed independent association with distant metastasis in our patients. We further analyzed the relative risk of distant metastasis according to age groups (Table 3). Distant metastasis and the 60 years and older age group were significantly associated (odds ratio, 17.8; 95% confidence interval [CI], 4.8 to 66.5; p < 0.001). The other older age groups (50 to 60 years and 40 to 50 years) were also significantly associated with distant metastasis, as compared with younger age groups (younger than 40 years) in multivariate analysis.
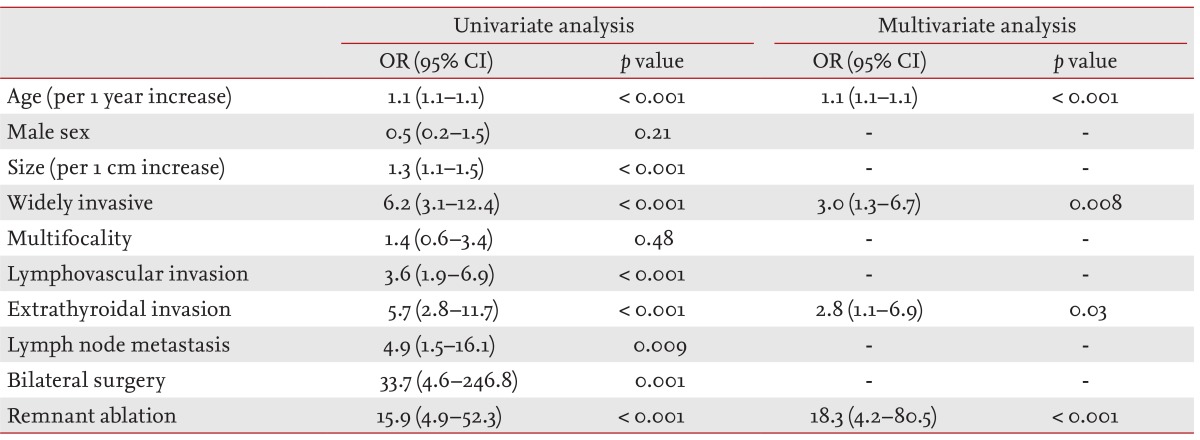
Clinicopathological paramenters associated with distant metastases in follicular thyroid carcinoma and Hurthle cell carcinoma
Clinicopathological parameters associated with recurrence
Of the 521 patients with FTC or HCC who did not have distant metastasis at the time of initial therapy, 14 patients (3%) had recurrent disease during a median of 6 years of follow-up. The DFS evaluation showed no significant difference between FTC and HCC groups (Fig. 2A). DFS was evaluated according to the presence of widely invasive cancer. Recurrence was more apparent in patients with WI-FTC, as compared with MI-FTC, although not significantly (p = 0.09) (Fig. 2B). DFS was not different in patients with wildly invasive and minimally invasive HCC (Fig. 2C).
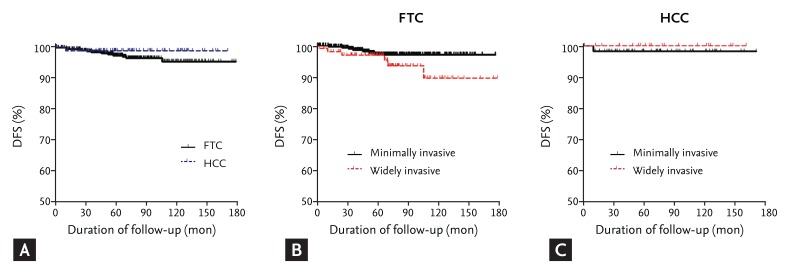
Disease-free survival (DFS) in follicular thyroid carcinoma (FTC) and Hurthle cell carcinoma (HCC) patients. (A) Comparison of DFS between FTC and HCC patients. (B) DFS of widely invasive type and minimally invasive type in FTC patients. (C) DFS of widely invasive type and minimally invasive type in HCC patients.
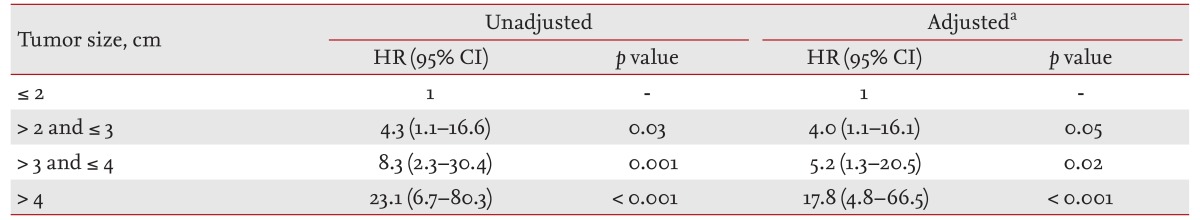
Larger tumor size, presence of lymphovascular invasion, extrathyroidal invasion and LN metastasis were significantly associated with recurrence based on univariate analysis (p = 0.005, p = 0.03, p = 0.01, and p < 0.001, respectively) (Table 4). However, only tumor size (hazard ratio [HR], 1.3; 95% CI, 1.1 to 1.6; p = 0.008) and LN metastasis (HR, 29.4; 95% CI, 2.7 to 99.4; p < 0.001) were independent risk factors for recurrence based on multivariate analysis. As compared with the smallest primary tumor size group (≤ 2 cm), the relative risk of recurrence in the larger tumor size groups (2 to 3 cm, 3 to 4 cm, > 4 cm) was significantly greater based on multivariate analysis (Table 5).
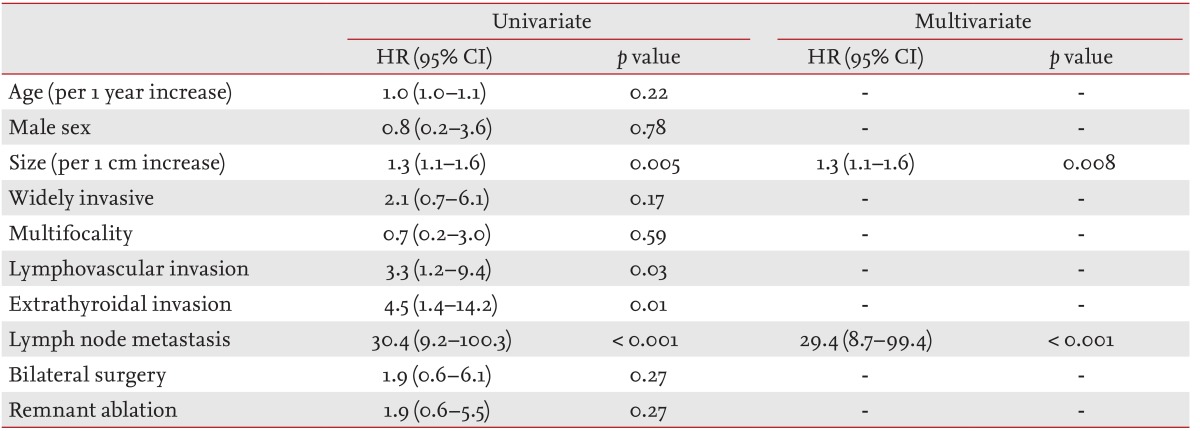
Clinicopathological parameters associated with recurrent disease in follicular thyroid carcinoma and Hurthle cell carcinoma patients
DISCUSSION
In this study, we evaluated a total of 563 patients with FTC or HCC. This is the first large multicenter cohort study in an iodine-sufficient area in Korea. HCC patients were older and had more lymphovascular invasions than FTC patients at the initial surgery. However, FTC and HCC patients had similar initial clinicopathological features. There was no significant difference in distant metastasis and DFS between the two groups. Older age, widely invasive type and extrathyroid extension were independent risk factors for distant metastasis in our study cohort. The relative risk of distant metastasis was 17.8-fold in patients older than 60 years, as compared with patients younger than 40 years. Primary tumor size and the presence of cervical LN metastasis were independent risk factors for recurrent FTC and HCC. There was no significant difference in recurrent FTC or HCC between minimally invasive and widely invasive types in our study cohort.
FNAC is the main diagnostic modality for evaluating thyroid nodules due to its accuracy and cost-effectiveness [14]. However, diagnosing FTC or HCC using FNAC is difficult because the risk of malignancy after the result of either follicular or Hurthle cell neoplasm is only 15% to 25% [15,16,17]. Therefore, clinicians attempt to determine diagnostic surgery according to clinical factors other than FNAC results, such as tumor size, the change in tumor size and patient age. In our study cohort, approximately 45% and 33% of patients with confirmed FTC and HCC, respectively, did not have malignant FNAC results. Diagnostic or therapeutic thyroidectomy was performed based on clinical decisions in the majority of these patients. Currently, a diagnostic marker useful for diagnosis of FTC or HCC is not available.
Previously, HCC was thought to have more aggressive clinical features with multifocal tumors, LN metastases and distant metastases compared with other DTCs [11,18,19]. In these studies, the frequency of distant metastases was 15% to 34% [11,18,19]. In a recent and large study using the Surveillance, Epidemiology and End Results (SEER) database, 3,311 patients with HCC were older and had larger tumor size, higher stage and higher disease-specific mortality, as compared with patients with other DTCs [20]. In this study, only 4.7% of patients had distant metastases [20]. In our study cohort, HCC patients were significantly older and had more lymphvascular metastases than FTC patients. However, the frequency of distant metastases and DFS after the first treatment was similar. Distant metastases were present in only 3% of our HCC patients. The frequency of distant metastases was lower in the recent large study and similar to our series [20]. Possibly, thyroid cancer was detected earlier in recent studies, contributing to the smaller number of distant metastases, as compared to historical studies.
Age was an important survival prognostic factor in the DTC and TNM staging system that includes age as a risk factor [9,10,19,21,22,23]. In a population-based study, the mortality HR was 6.35 in DTC patients over 50 years of age, compared with younger patients [20]. Older age was an independent risk factor for distant metastasis in our study cohort, which is in agreement with a previous study. When deciding on an initial therapeutic strategy for FTC and HCC patients, age should be considered as a risk factor.
The MI-FTC and WI-FTC categories defined by WHO classification are generally accepted [9]. However, definition of the capsular invasion extent varies between pathologists. D'Avanzo et al. [22] proposed that MI-FTC should be categorized as FTC with capsular invasion only and moderately invasive FTC should include angioinvasion with or without capsular invasion to emphasize the prognostic importance of vascular invasion. In our study cohort, lymphovascular invasion was not a significant prognostic factor associated with distant metastases or recurrent disease in multivariate analysis and showed significance as a prognostic factor only in the univariate analysis. Therefore, we analyzed our study subjects within minimally invasive and widely invasive subclassifications. The extent of capsular invasion was an important risk factor for distant metastasis and survival in previous studies [18,19,21,22,23,24]. In our study subjects, the widely invasive type of tumor was an important risk factor for distant metastases in FTC and HCC patients. The extent of capsular invasion was a marginally significant risk factor when estimating the recurrence of FTC, even though our median follow-up period was only 6 years. However, therapeutic modalities such as bilateral thyroid surgery and radioiodine ablation therapy could possibly influence DFS between widely invasive and minimally invasive tumors.
This study had several limitations. First, the patient follow-up period after their initial treatment was relatively short and was limited to evaluating cancer-specific survival in FTC and HCC patients. Second, this retrospective study included patients from four tertiary referral centers and, therefore, the possibility of selection bias exists. Additionally, therapeutic strategies between centers may vary. Third, the clinical importance of cervical LN metastasis in FTC and HCC patients could be underestimated because we could not classify Nx from N0 in this retrospective study. However, this is the first multicenter cohort study describing the clinical behaviors of FTC and HCC in Korea. Although HCC patients were older and had more lymphovascular invasions, clinicopathological features of FTC and HCC were relatively comparable. The age of patients, wide invasiveness and extrathyroidal invasion were independent risk factors for predicting distant metastases in FTC and HCC patients in the present study.
KEY MESSAGE
Hurthle cell carcinoma (HCC) patients were older and had more lymphovascular invasions than follicular thyroid carcinoma (FTC) patients.
Distant metastasis and recurrence were not significantly different between FTC and HCC patients.
The patient's age, wide invasiveness and extrathyroidal invasion were independent risk factors for predicting distant metastases in FTC and HCC patients.
Acknowledgments
This study was supported by a grant (No. 2013-374, 2013-582) from the Asan Institute for Life Sciences, Seoul, Korea.
Notes
An abstract (ITC2010-1183) covering a part of this article was presented at the 14th International Thyroid Congress, September 15, 2010, Paris, France.
No potential conflict of interest relevant to this article was reported.